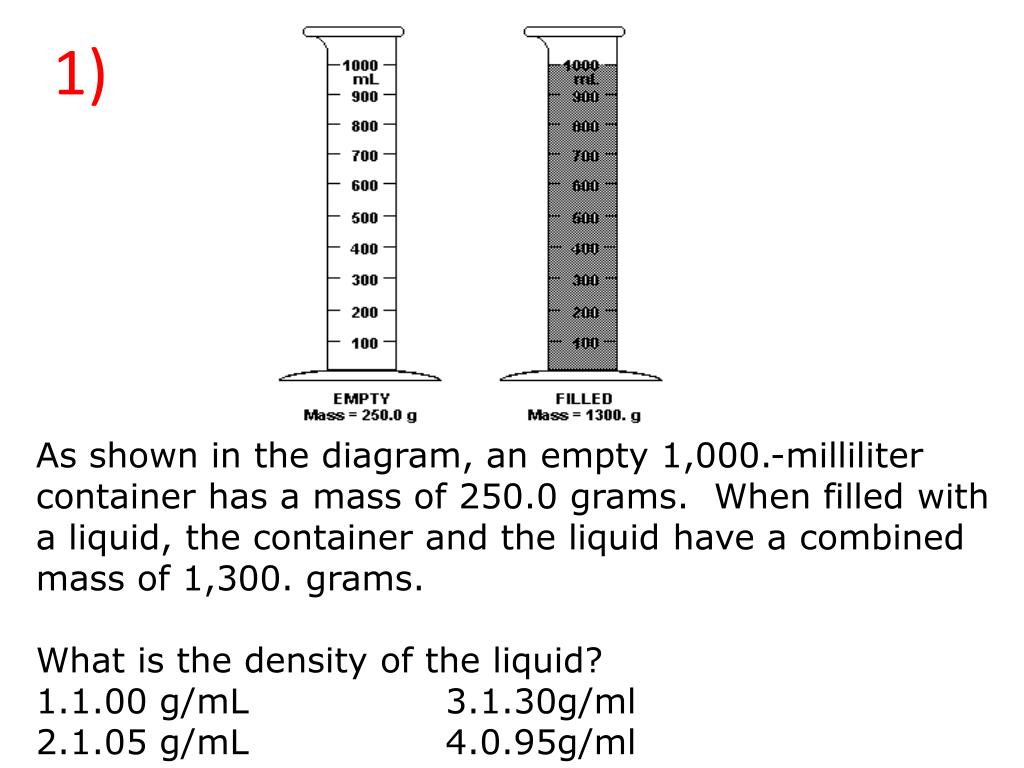
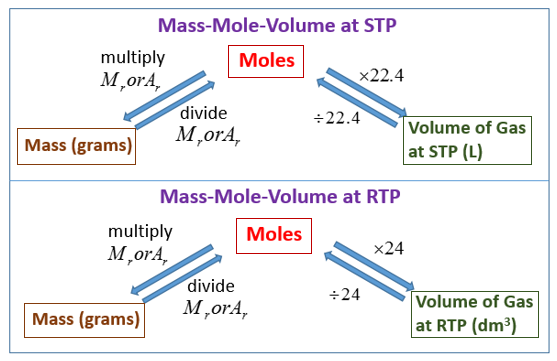
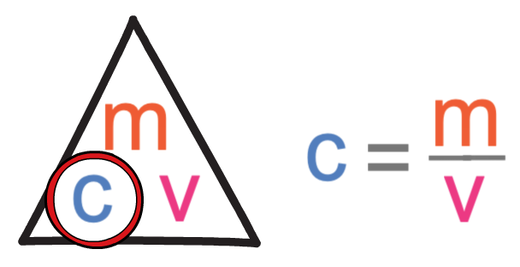An empty beaker weighs 42.75 grams. When completely filled with water, the beaker and its contents have a total mass of 399.95 grams. What volume does the beaker hold? Use d =

SOLVED: For the masses and volumes indicated, calculate the density in grams per cubic centimeter. (a) mass = 454.1 g; volume = 291 cm3 =g/cm3 (b) mass = 0.38 lb; volume =

SOLVED: What is the volume (in cm³) of 3.045 grams of magnesium? (Density 1.74 g/cm³) 1.8 cm³? 0 0.57 cm³? 1.75 cm³? 1.7 cm³? 0.571 cm³?
Solved: Gathered Data: Density = 1.80 g/mL Volume=0.32 L Required: mass in grams Solution: Mass=(D [Chemistry]