
States of Matter & Bond Strength –Gas –Gas = Molecules not bonded to one another; move independently. Takes the volume and shape of its container. –Liquid. - ppt download
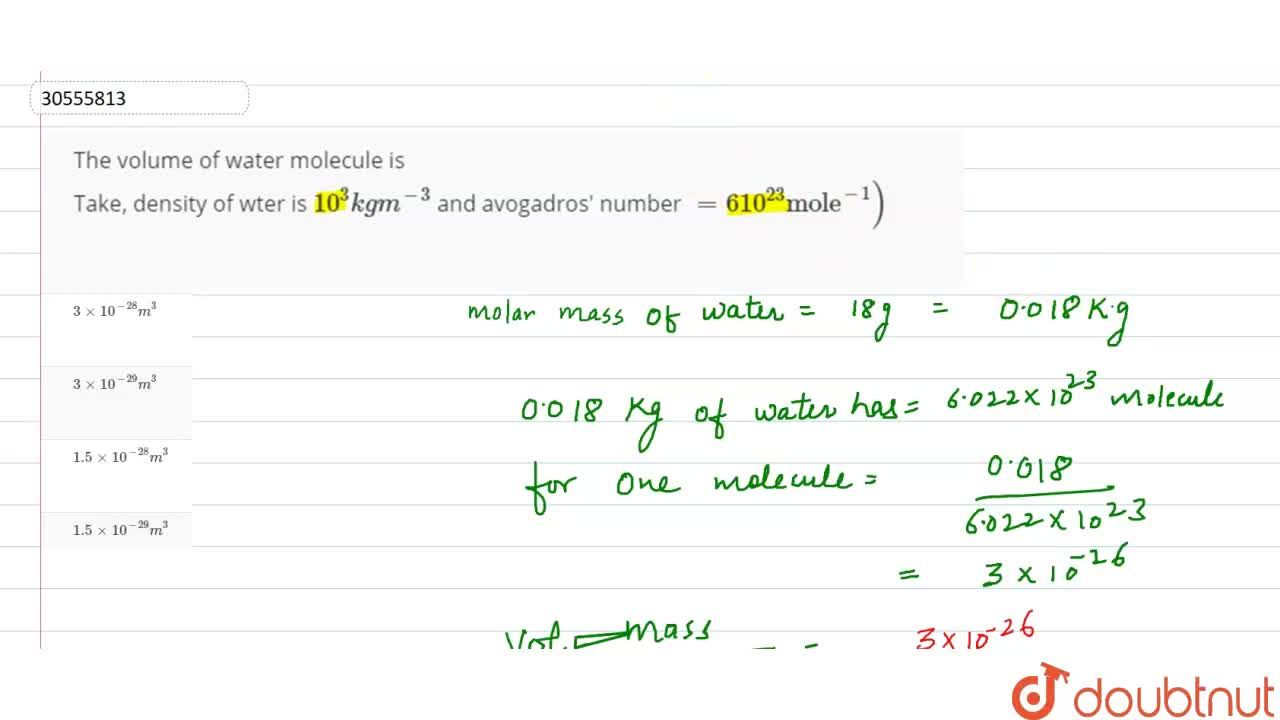
The volume of water molecule is Take, density of wter is 10^(3) kg m^(-3) and avogadros' number =610^(23) "mole"^(-1))

SOLVED: Question 9 1pts The volume of a single molecule of water is 2.99 * 10-23 mL.For a sample of gaseous water at 1.00 atm and 150 %C,what fraction of the container's
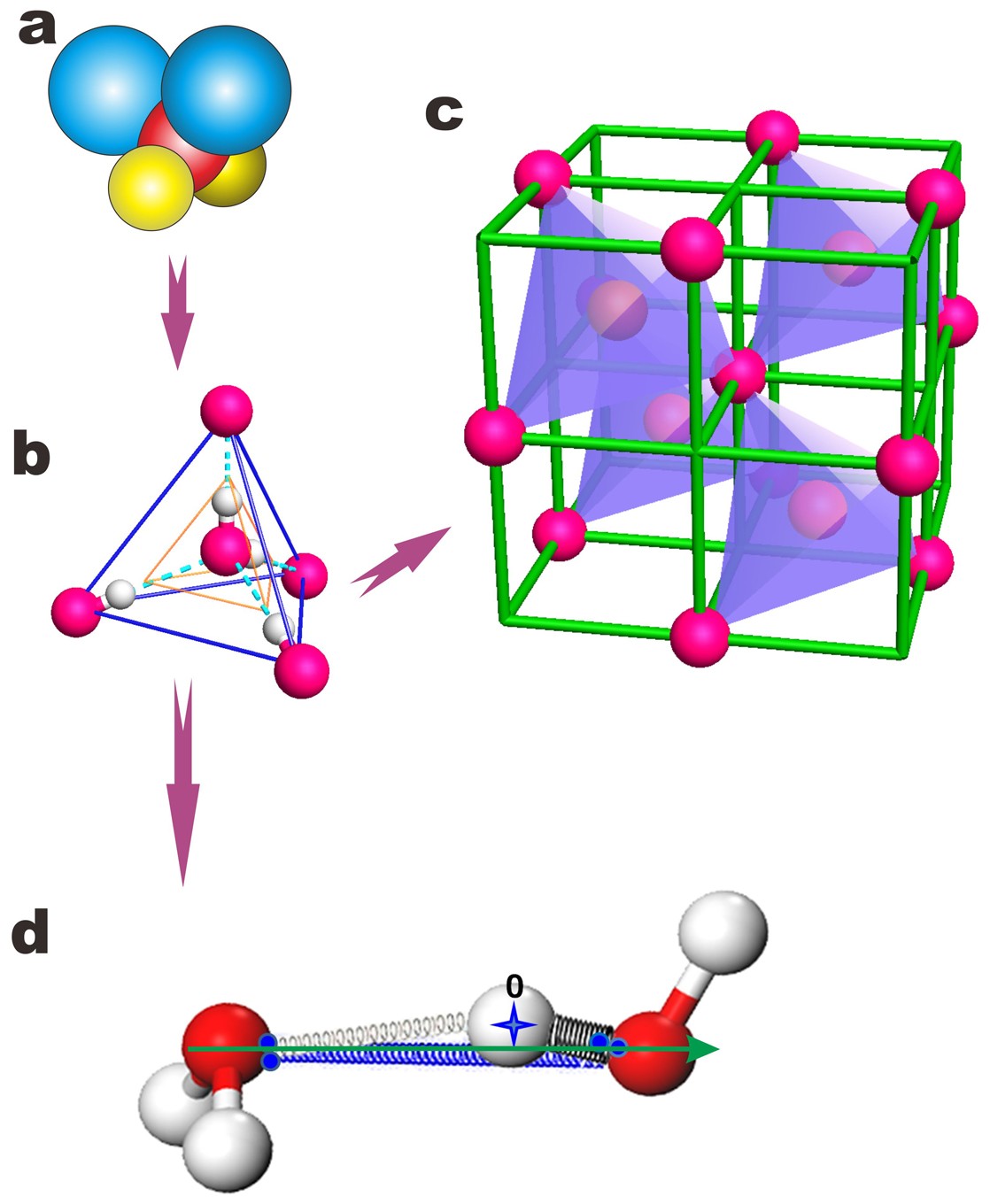
Size, separation, structural order and mass density of molecules packing in water and ice | Scientific Reports

Calculate- (a) Actual volume of 1 molecule of water (b) Radius of water molecule assuming it to be spherical - Chemistry - Some Basic Concepts of Chemistry - 10257567 | Meritnation.com

Calculate (i) the volume of one molecule of water. (ii) the radius of a water molecule assuming the molecule to be spherical. (Given that the density of water is 1 g/cm^3.


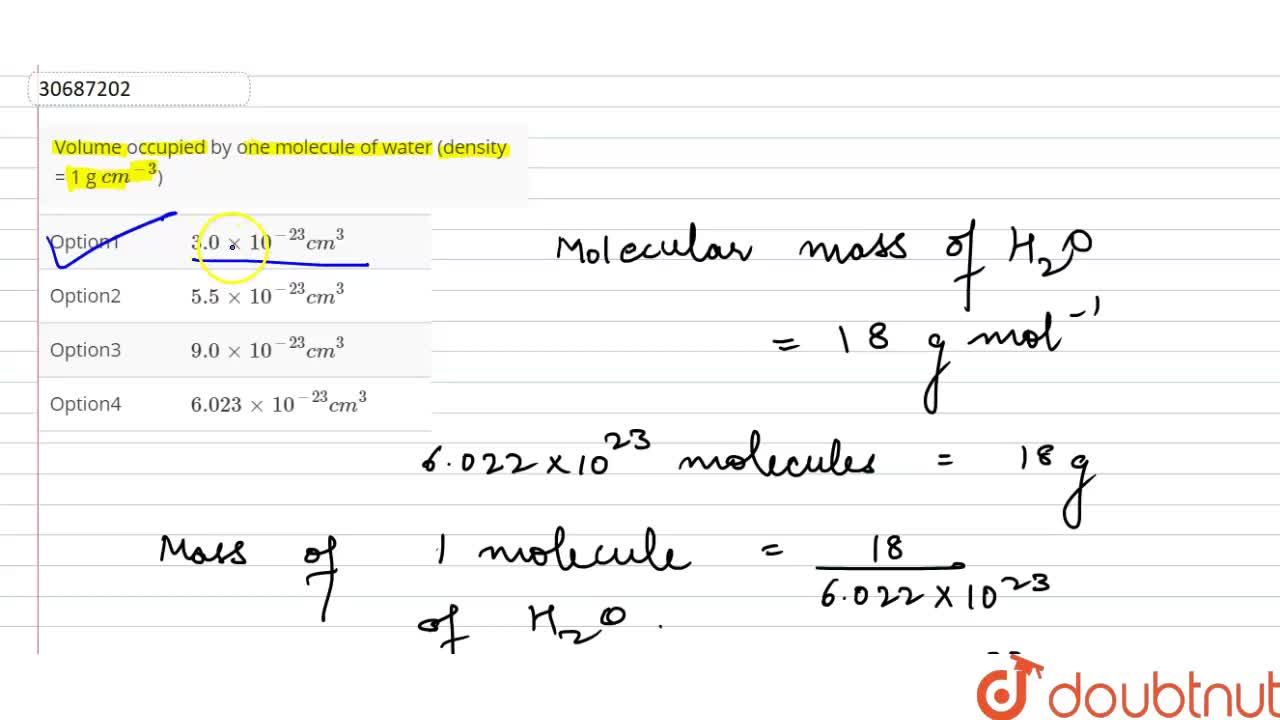











![Volume occupied by one molecule of water is:[density = 1 g cm^-3] Volume occupied by one molecule of water is:[density = 1 g cm^-3]](https://dwes9vv9u0550.cloudfront.net/images/11787771/5012c0ab-92d4-445f-8570-631fbb87fc75.jpg)


