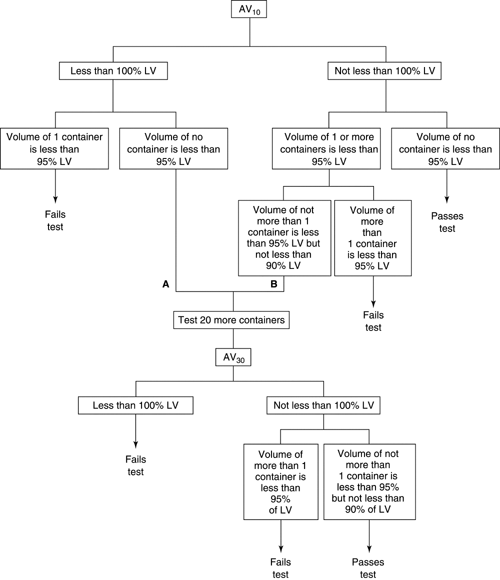
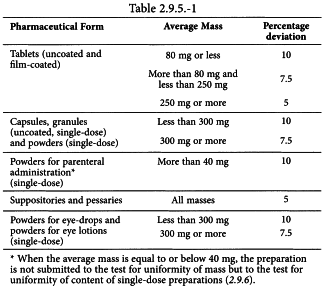
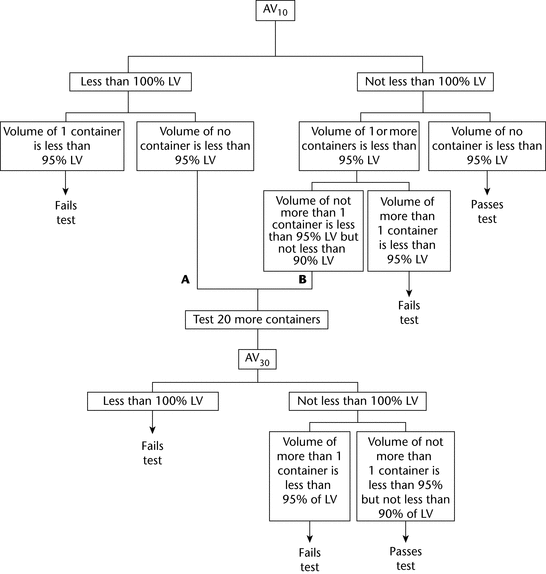
5.6 EXTRACTABLE VOLUME FOR PARENTERAL PREPARATIONS … / 5-6-extractable- volume-for-parenteral-preparations.pdf / PDF4PRO

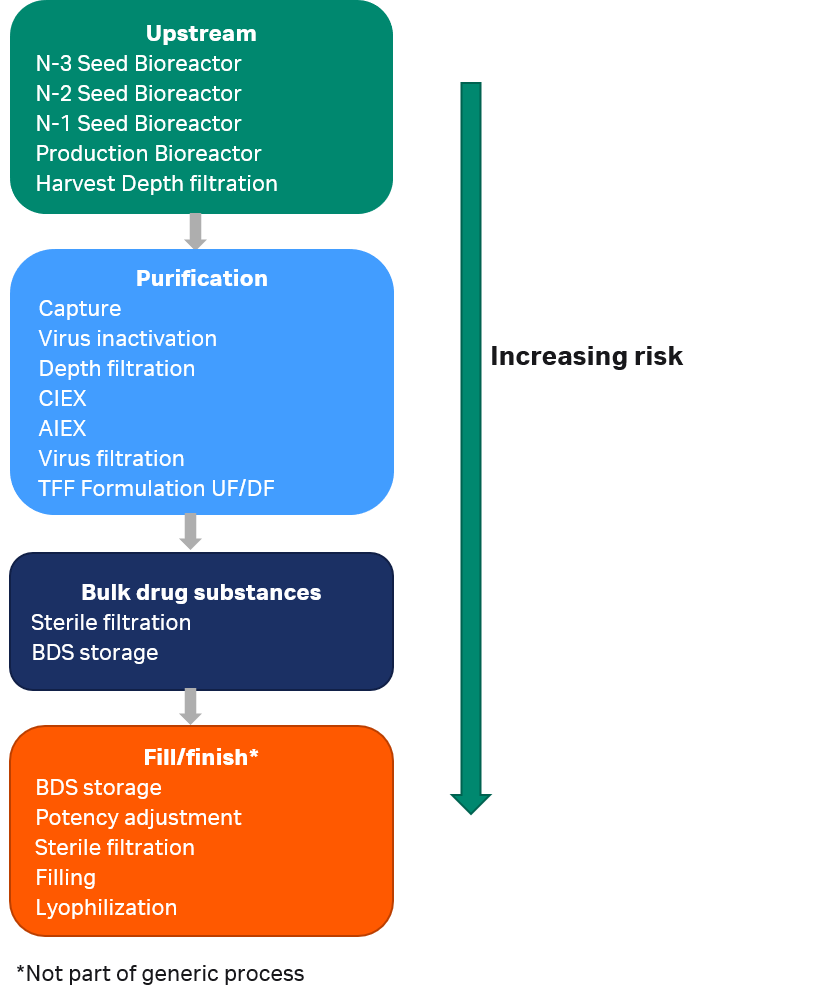
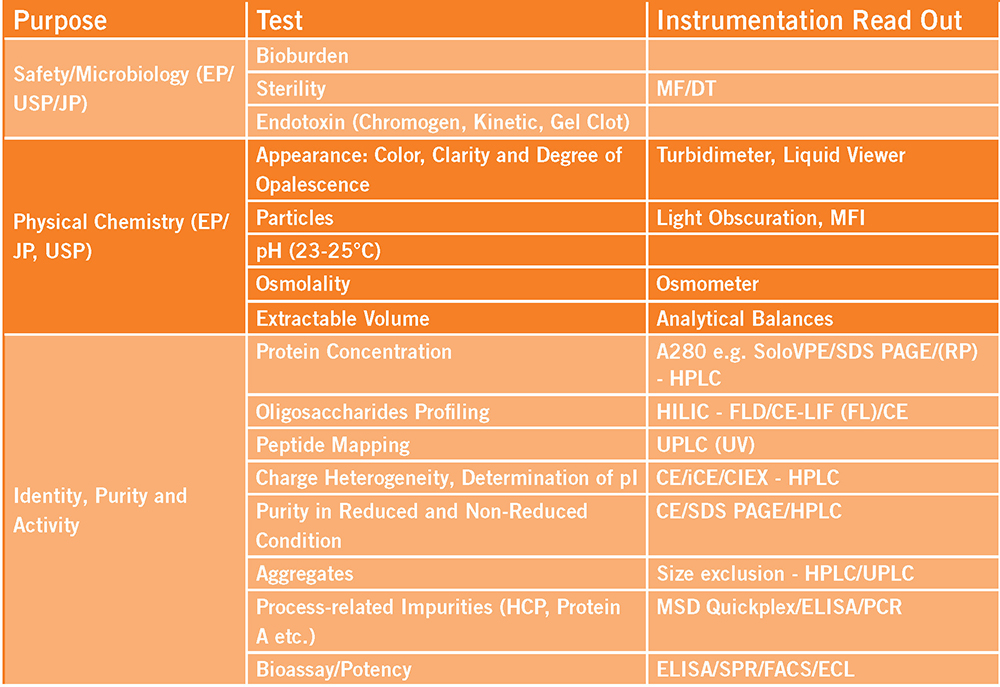
Using extractables data from single-use components for extrapolation to process equipment-related leachables: The toolbox and justifications - ScienceDirect