A2LA Cert. No. 5004.02) 09/30/2022 Page 1 of 5 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2017 MARTIN BAUER INC – US LABORATORY
PDF) From heavy metals testing to the measurement of elemental impurities in pharmaceuticals: Over 100 years in making the change
From Heavy Metals Testing to the Measurement of Elemental Impurities in Pharmaceuticals: Over 100 Years in Making the Change
From Heavy Metals Testing to the Measurement of Elemental Impurities in Pharmaceuticals: Over 100 Years in Making the Change
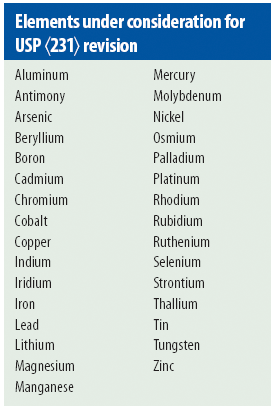
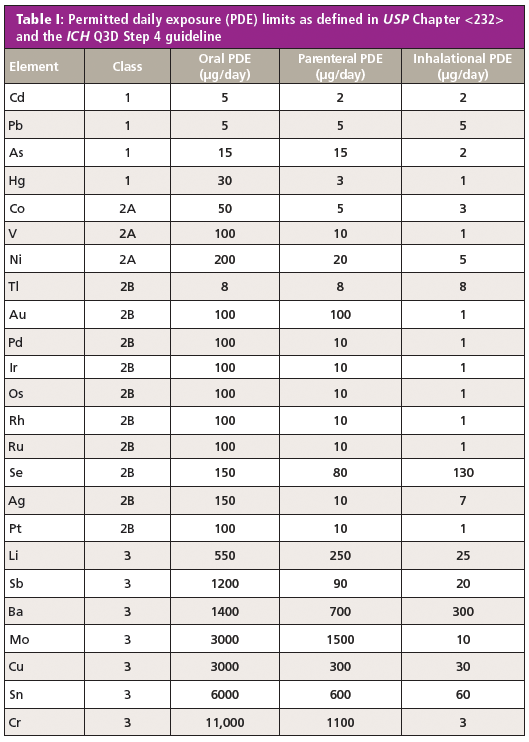
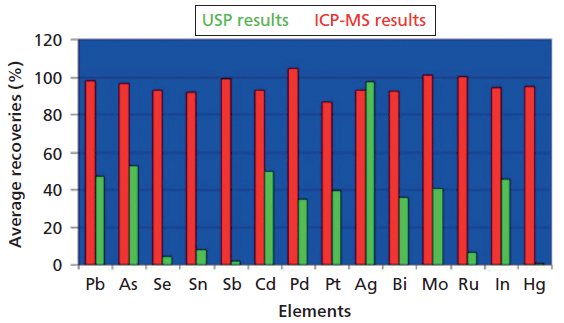
ELEMENTAL IMPURITY ANALYSIS IN PHARMACEUTICALS ICH and USP tests for elemental impurities provide better indication of potential

Evolving from the 100 Year Old USP Method to USP - SGS Harrisburg, PA | Heavy Metals and Vitamins Analysis, Dietary Supplements and Dietary Ingredients, Cosmetics, Lead Testing, Elemental Trace Metals, Inorganic Impurities Analysis