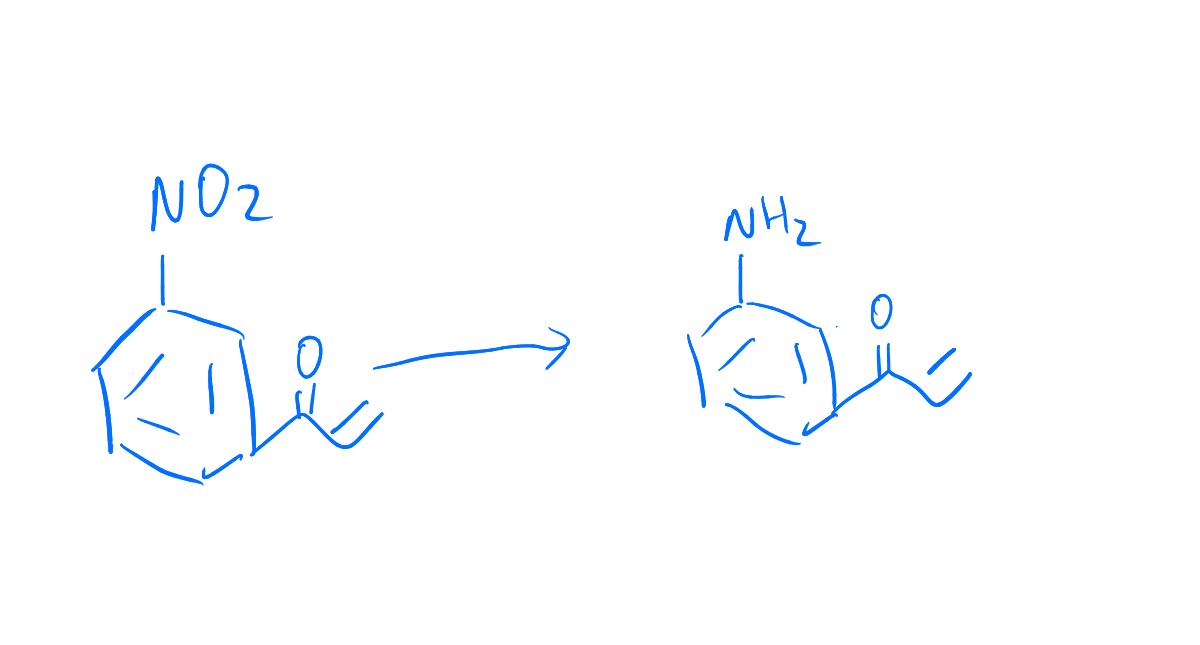
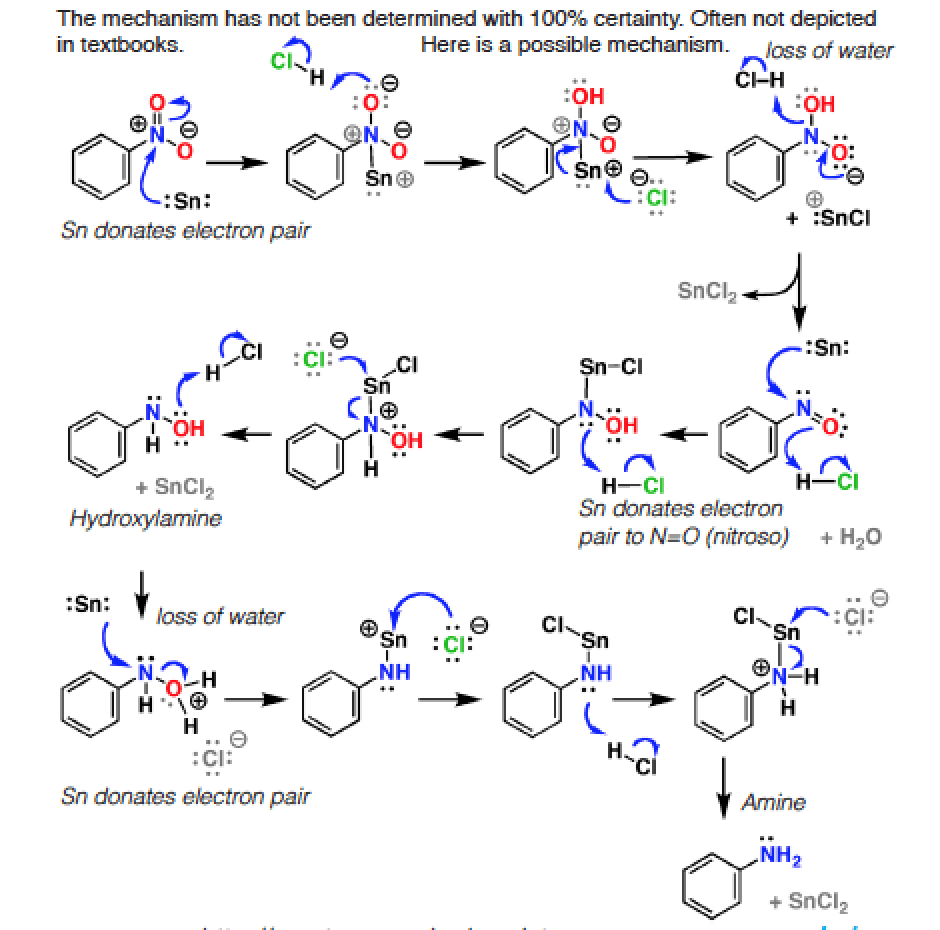
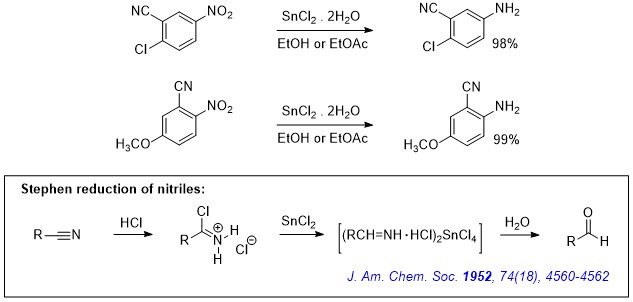
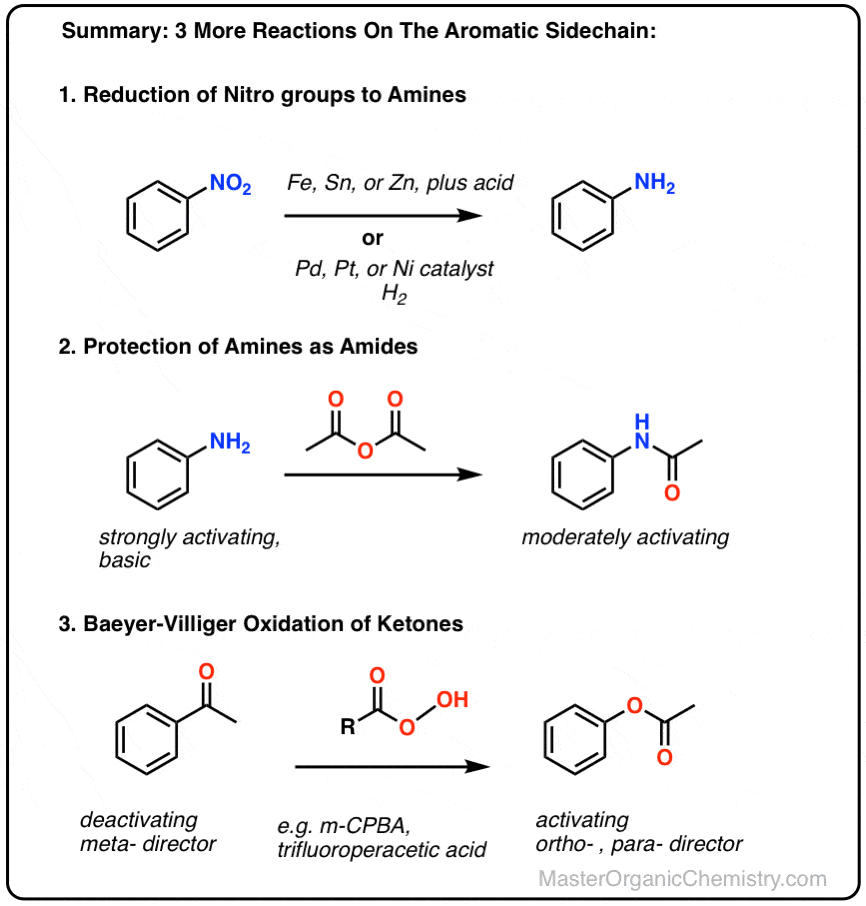
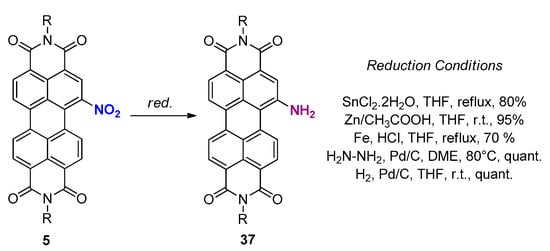
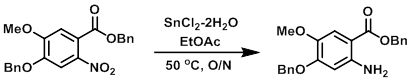organic chemistry - Pyridine synthesis by tin(II) chloride reduction of 5-nitronorbornene - Chemistry Stack Exchange
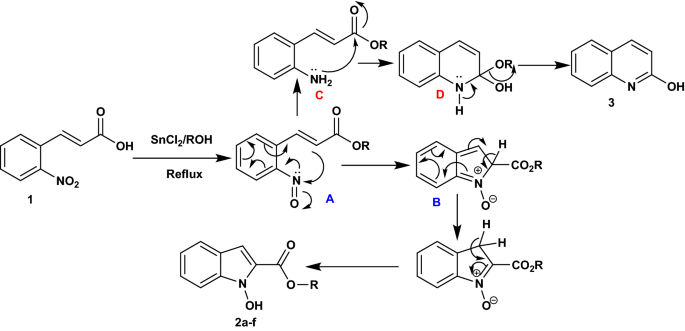
Efficient One-Pot Synthesis of Indolhydroxy Derivatives Catalyzed by SnCl2, DFT Calculations and Docking Study | Chemistry Africa

organic chemistry - Selective reduction of nitro group to amine, in benzene ring containing nitrile? - Chemistry Stack Exchange
![Specific Features of the Reduction of 7-Nitro-9-R-2,3-dihydroimidazo[1,2-a]benzimidazoles with SnCl2 in Hydrochloric Acid | Russian Journal of Organic Chemistry Specific Features of the Reduction of 7-Nitro-9-R-2,3-dihydroimidazo[1,2-a]benzimidazoles with SnCl2 in Hydrochloric Acid | Russian Journal of Organic Chemistry](https://media.springernature.com/lw685/springer-static/image/art%3A10.1134%2FS1070428022050062/MediaObjects/11178_2022_3668_Sch1_HTML.gif)
Specific Features of the Reduction of 7-Nitro-9-R-2,3-dihydroimidazo[1,2-a]benzimidazoles with SnCl2 in Hydrochloric Acid | Russian Journal of Organic Chemistry

Synthesis of 2,5-disubstitued benzimidazole using SnCl2-catalyzed reduction system at room temperature - ScienceDirect
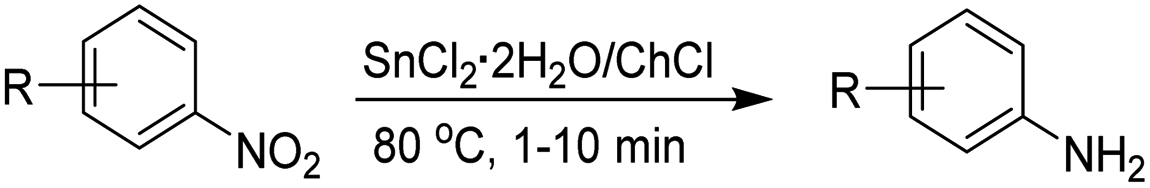
Tin( ii ) chloride dihydrate/choline chloride deep eutectic solvent: redox properties in the fast synthesis of N -arylacetamides and indolo(pyrrolo)[1 ... - RSC Advances (RSC Publishing) DOI:10.1039/D0RA06871C

A DFT study of reduction of nitrobenzene to aniline with SnCl2 and hydrochloric acid - Yamabe - 2016 - Journal of Physical Organic Chemistry - Wiley Online Library