Chemistry – Metal carbonate and hygrodencarbonates - Acids, bases and salts - Part 2 -English - YouTube
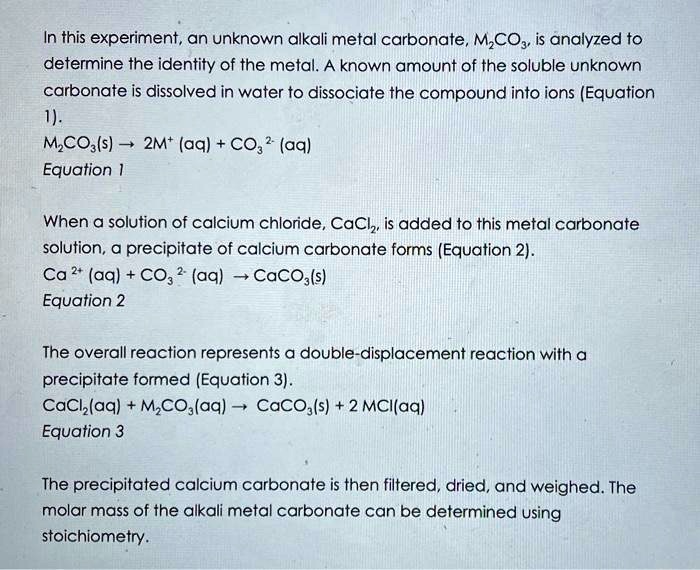
SOLVED: In this experiment, an unknown alkali metal carbonate, MCOz, is analyzed to determine the identity of the metal. A known amount of the soluble unknown carbonate is dissolved in water to

1.725 g of a metal carbonate is mixed with 300 mL of N/10 HCI. 10 mL of N/2 sodium hydroxide were required to neutralise excess of the acid. Calculate the equivalent mass
6.10 g metal carbonate on heating produces 5.6 gram of metal oxide,then equivalent weight of metal carbonate is?

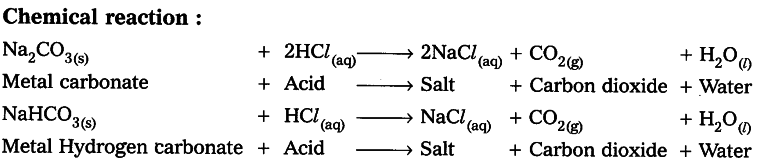
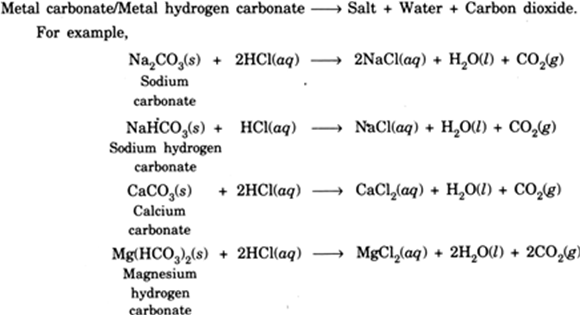
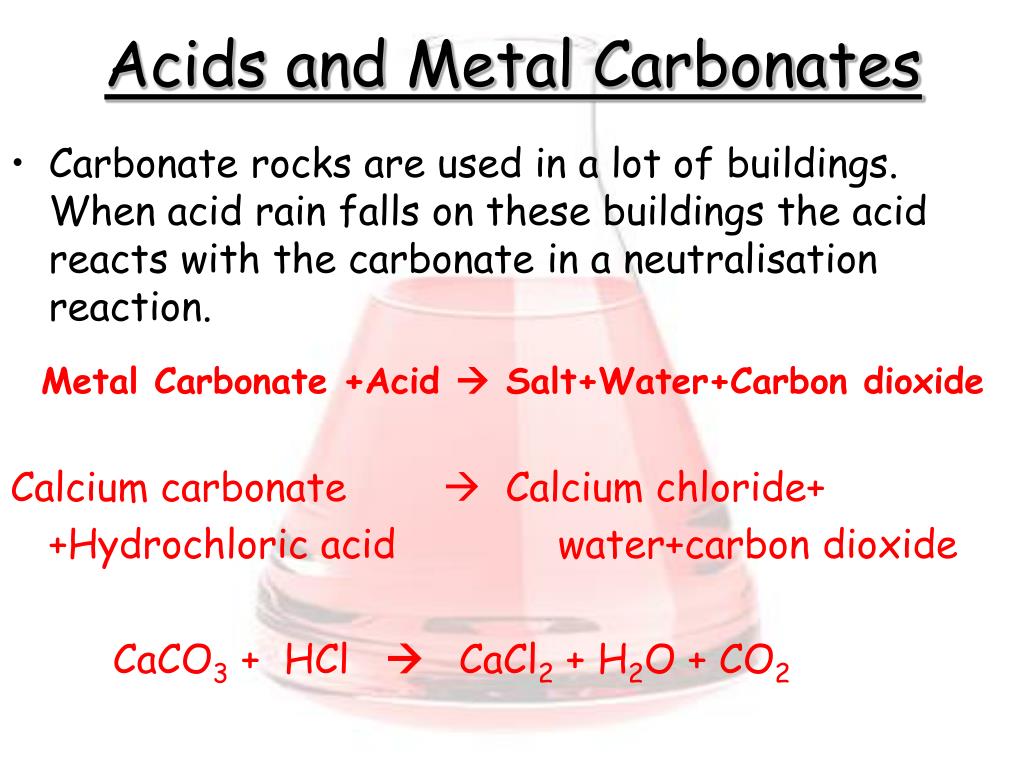
how do metal carbonates and metal hydrogen carbonates react with acids ? give their chemical equations . - Brainly.in
1.725 g of a metal carbonate is mixed with 300 ml of N/10 HCl. 10 ml of N/2 sodium hydroxide were required to neutralise excess of acid. Calculate the equivalent mass of metal carbonate.





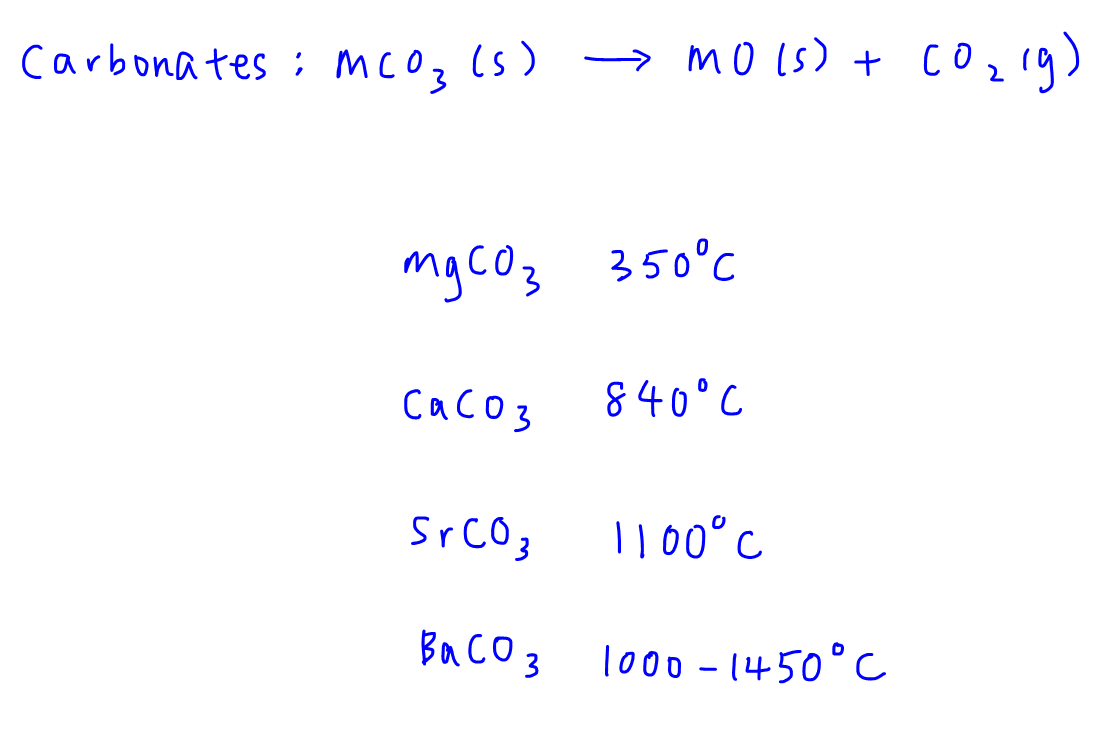
.png)