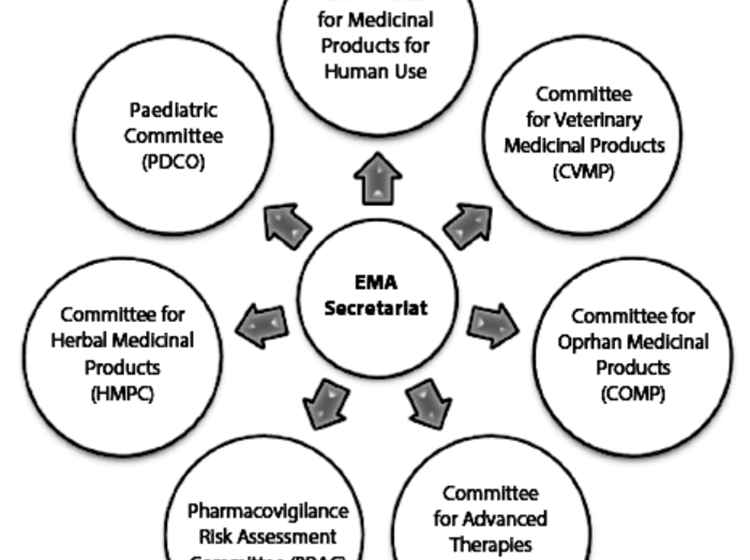
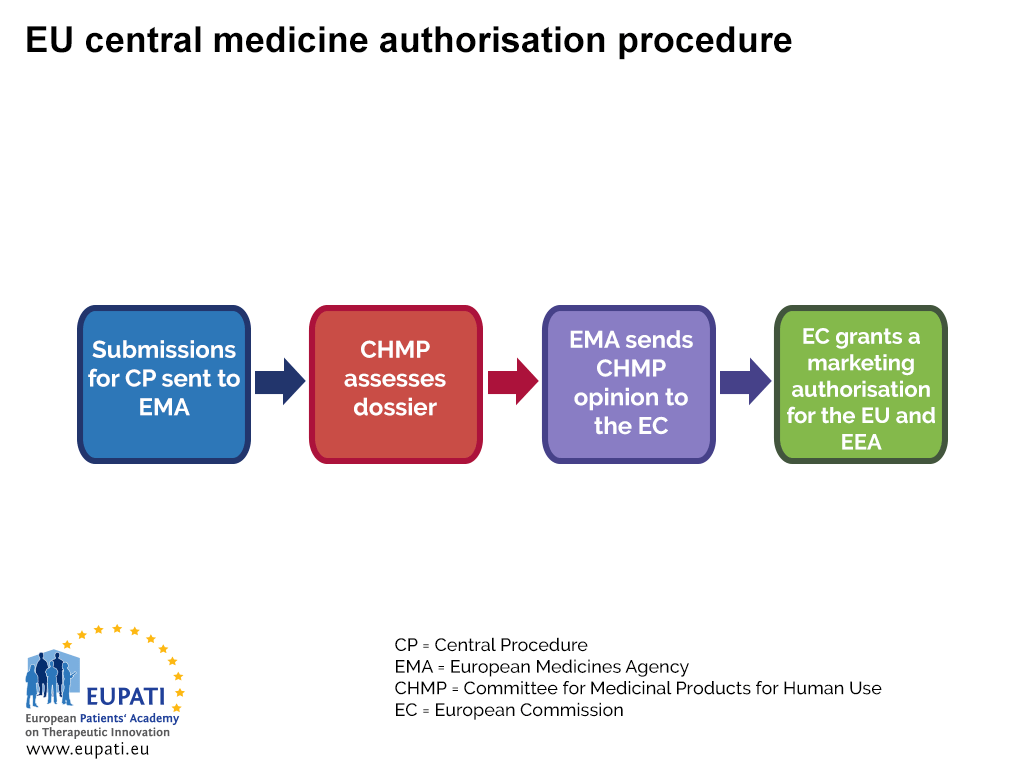
EMA's Committee for Medicinal Products for Human Use (CHMP) - News, Articles etc. - European Pharmaceutical Review
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 18-21 March 2024 | European Medicines Agency

Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 7-10 November 2022
EMA Committee for Medicinal Products for Human Use (CHMP) - News, Articles etc. - European Pharmaceutical Review
Live biotherapeutic products: the importance of a defined regulatory framework | Experimental & Molecular Medicine

Pharmaceutical Legislation On Notice To Applicants And Regulatory Guidelines For Medicinal Products by PepGra CRO - Issuu