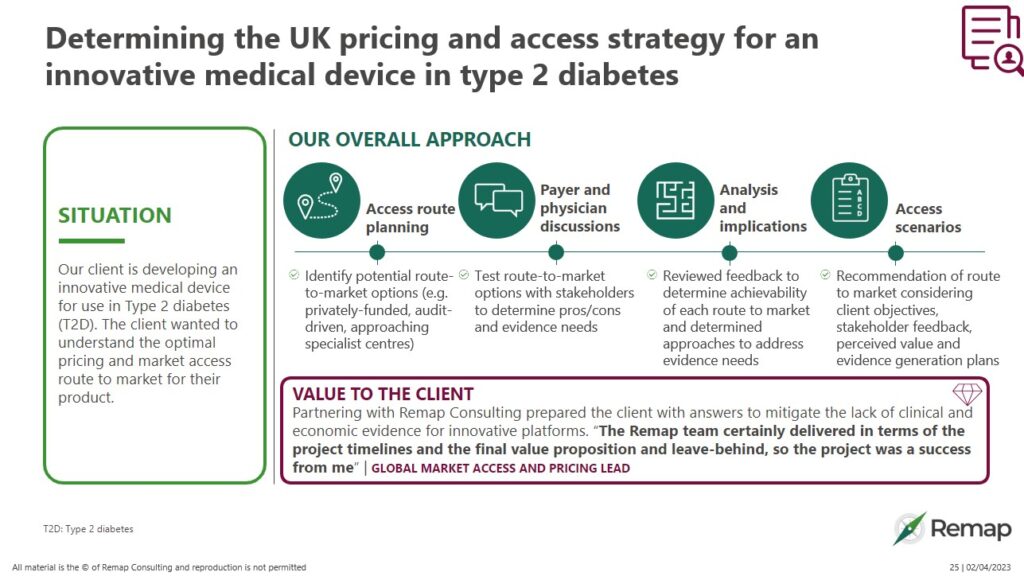
Determining the UK pricing and access strategy for an innovative medical device in type 2 diabetes - Remap Consulting

How to create the best market access and reimbursement strategy for your medical device | Definitive Healthcare

Buy Medical Devices and IVDs: Market Access under the new EU Regulations - compact course for study, project and job Book Online at Low Prices in India | Medical Devices and IVDs:

Market access in Japan before and after the Pharmaceutical and Medical... | Download Scientific Diagram

Understanding CE Marking for Medical Devices: Ensuring Compliance and Market Access | by Webfrog IT Services Team | I3CGLOBAL Blogs | Medium