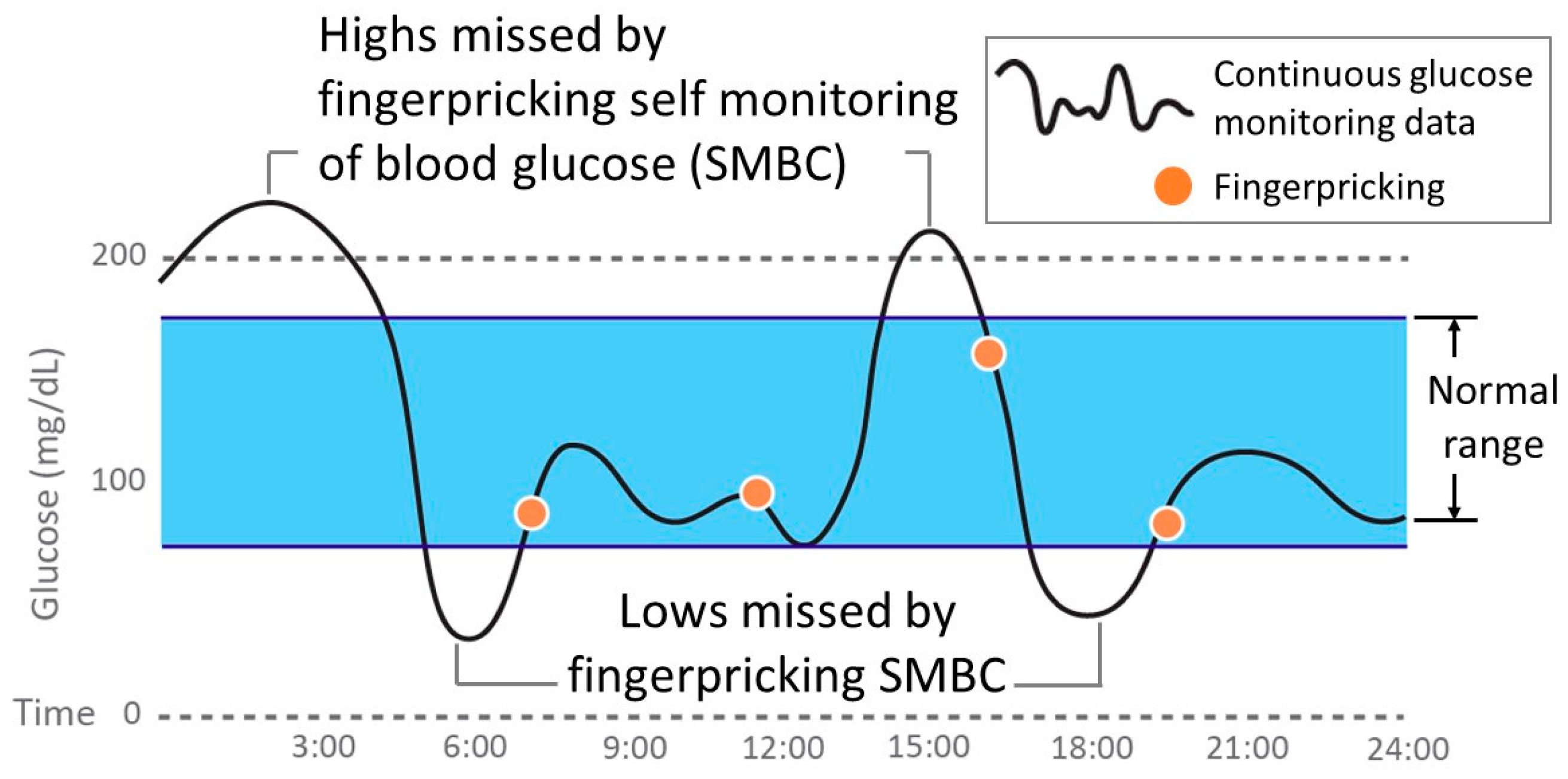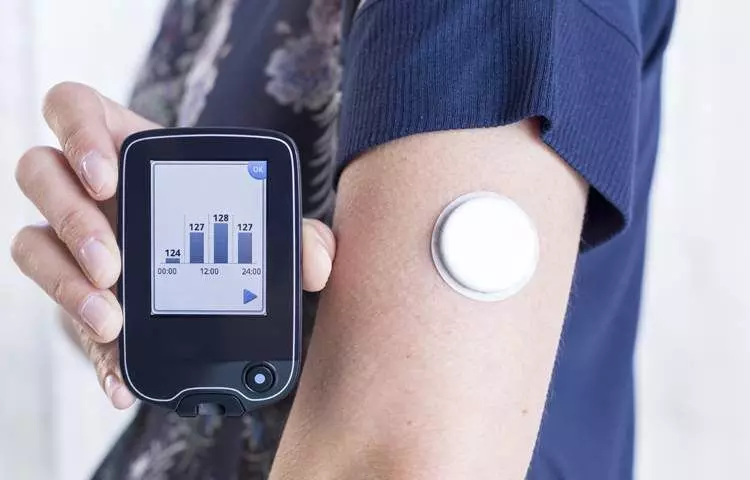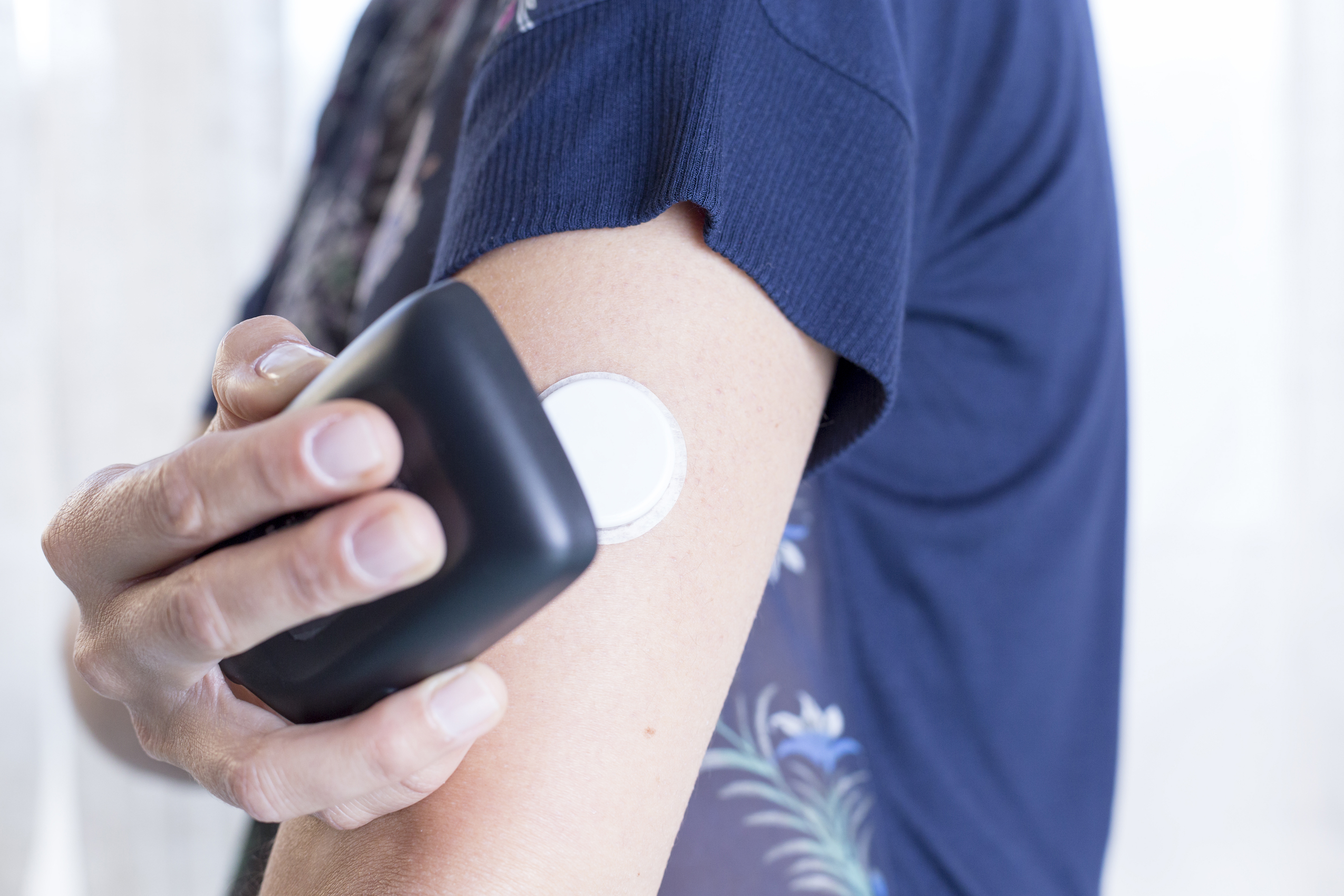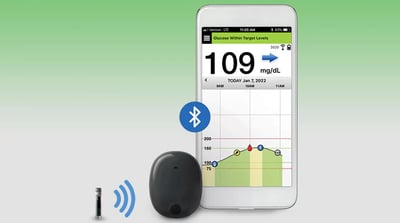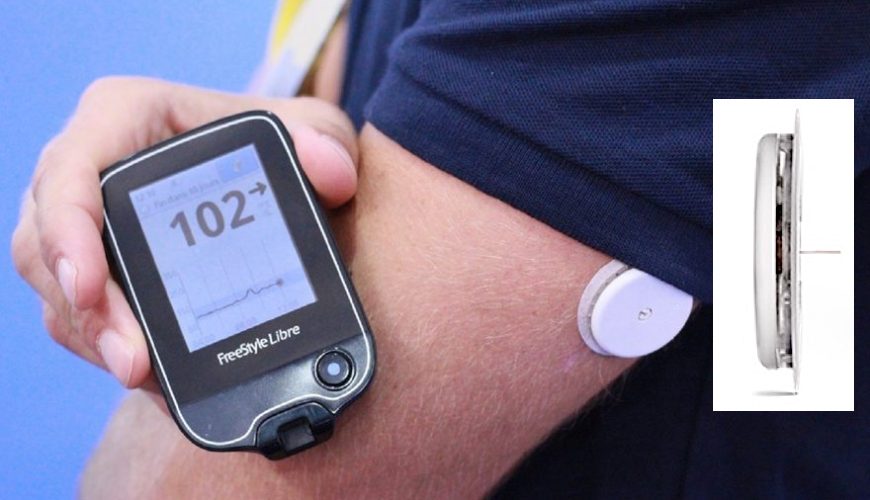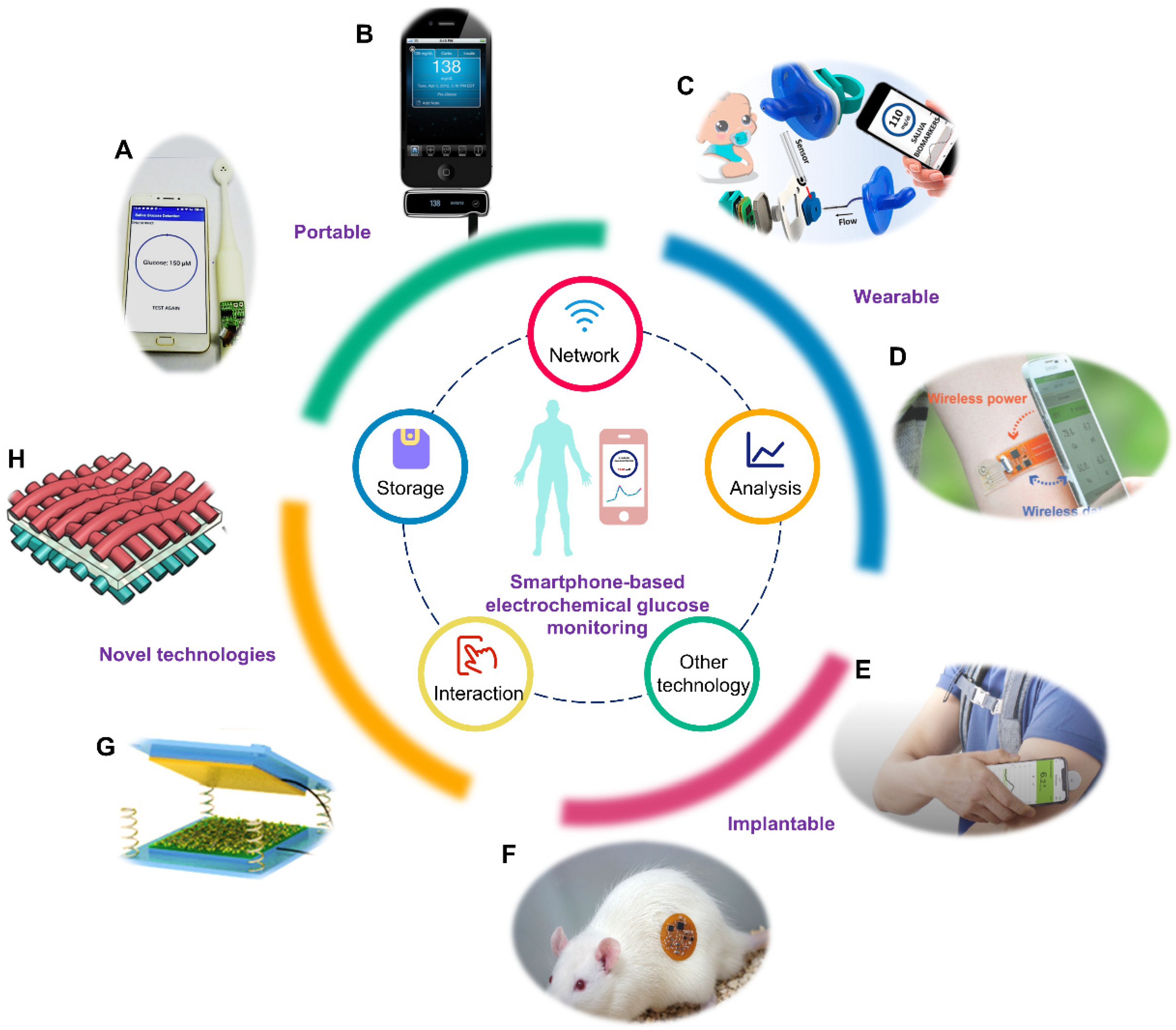
Sensors | Free Full-Text | Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review
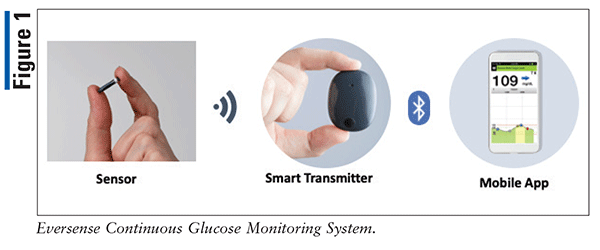
Insight into continuous glucose monitoring: from medical basics to commercialized devices | Microchimica Acta

Minimally Invasive Implant Type Electromagnetic Biosensor for Continuous Glucose Monitoring System: In vivo Evaluation - IEEE Transactions on Biomedical Engineering (TBME)
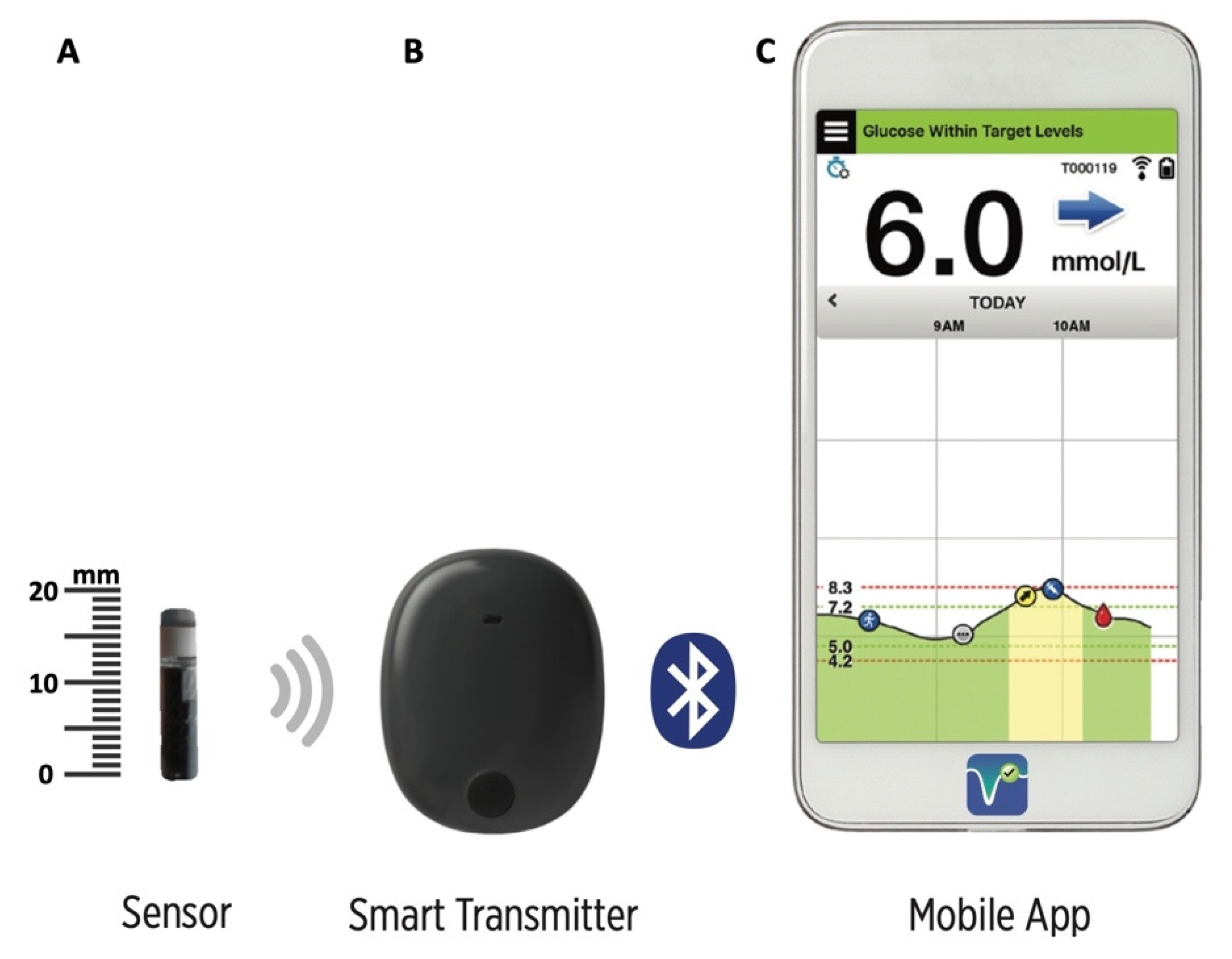
Animals | Free Full-Text | Clinical Use of a 180-Day Implantable Glucose Monitoring System in Dogs with Diabetes Mellitus: A Case Series

Applied Sciences | Free Full-Text | Design of a Sandwich Hierarchically Porous Membrane with Oxygen Supplement Function for Implantable Glucose Sensor

Sensors | Free Full-Text | Batteryless, Miniaturized Implantable Glucose Sensor Using a Fluorescent Hydrogel

GLUCOTRACK ANNOUNCES EARLY ACCURACY DATA FOR ITS IMPLANTABLE CONTINUOUS GLUCOSE MONITOR – Mining Discovery

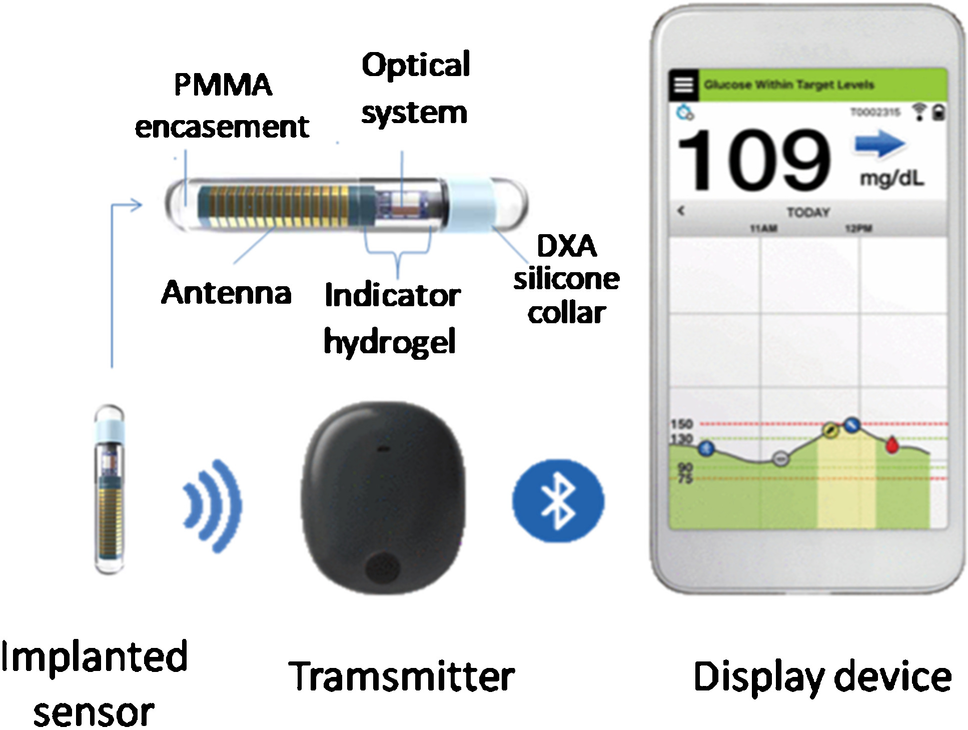
Eversense Ò implantable glucose sensor. DXA, dexamethasone acetate;... | Download Scientific Diagram

Could Implantable Glucose Sensors be a Viable Option for Monitoring Blood Sugar? | by Diabetes Research Connection | Medium
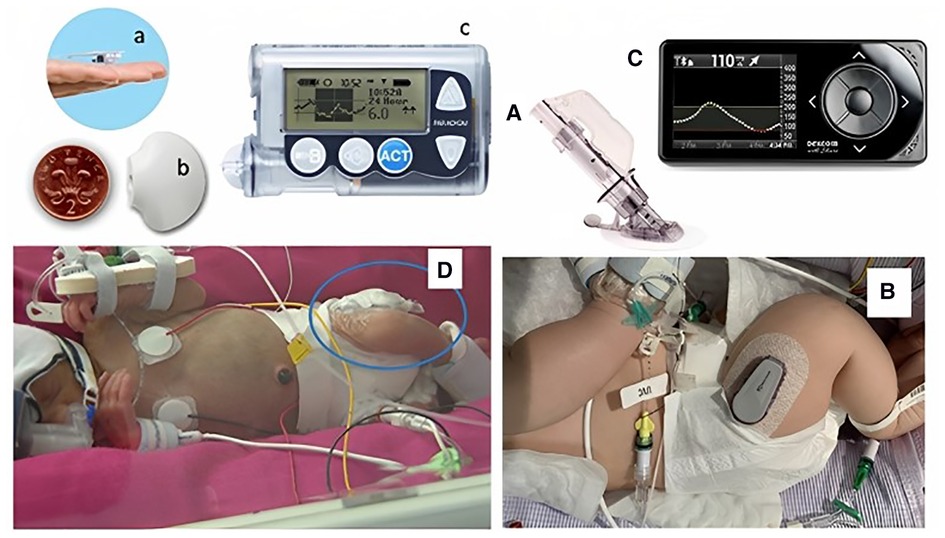
Frontiers | Should continuous glucose monitoring be used to manage neonates at risk of hypoglycaemia?

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News