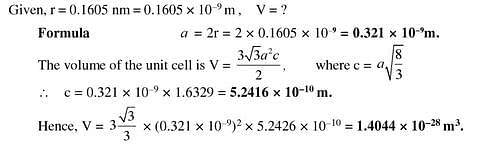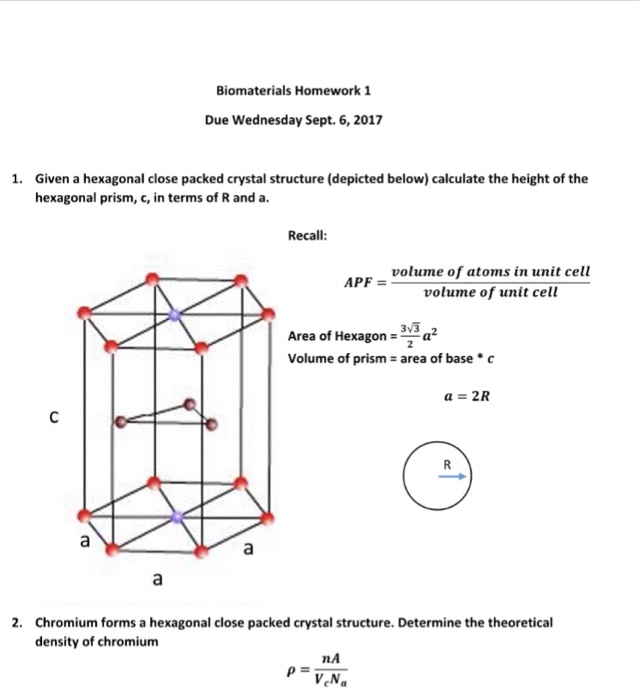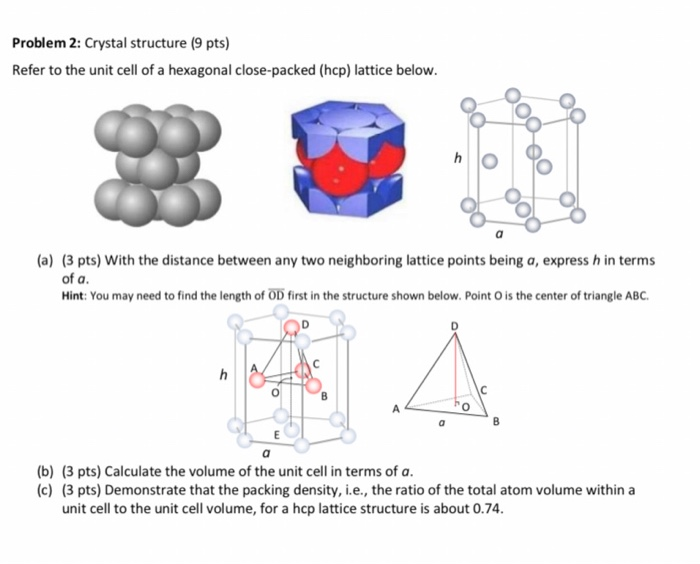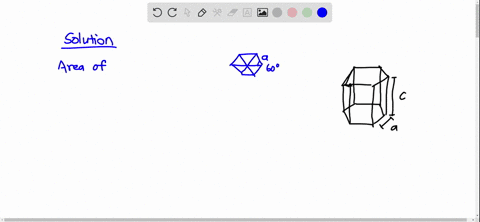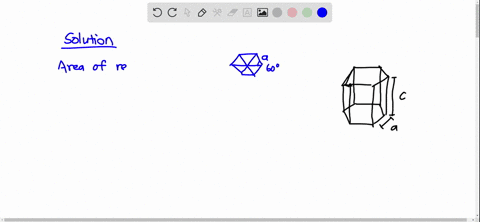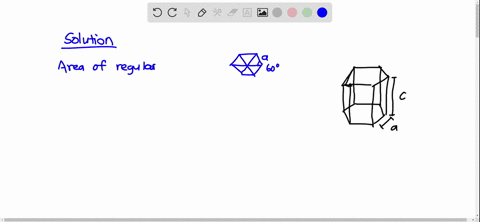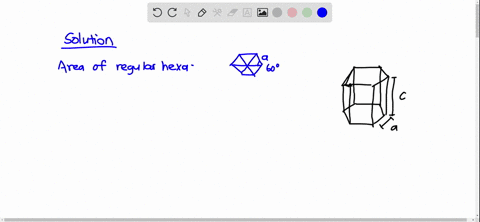
SOLVED: Cobalt has an HCP crystal structure, an atomic radius of 0.1253 nm, and a c / a ratio of 1.623 Compute the volume of the unit cell for Co. | Numerade
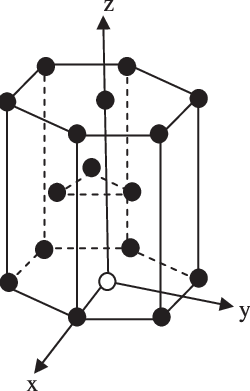
Volume of HCP unit cell is:A)$\\text{ }24\\sqrt{2}\\text{ }{{\\text{r}}^{3}}$ B)$\\text{ }8\\sqrt{2}\\text{ }{{\\text{r}}^{3}}$C)$\\text{ }16\\sqrt{2}\\text{ }{{\\text{r}}^{3}}$D)$\\text{ 24}\\sqrt{\\text{3}}\\text{ }{{\\text{r}}^{\\text{3}}}$
The volume of this hcp unit cell is (A) 24√2r^3 (B) 16√2r^3 - Sarthaks eConnect | Largest Online Education Community

What is the volume in (nm^3) of the beryllium unit cell? Beryllium has a hexagonal closed packed unit cell with 0.22856 nm and 0.3582 nm. | Homework.Study.com

A common distortion of the hcp lattice produces a doubling of the cell... | Download Scientific Diagram
What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering

Cohesive energy as a function of atomic volume of HCP unitcell using... | Download Scientific Diagram

Difference Between Primitive Hexagonal Unit Cell and Hexagonal Closed Packing | Compare the Difference Between Similar Terms