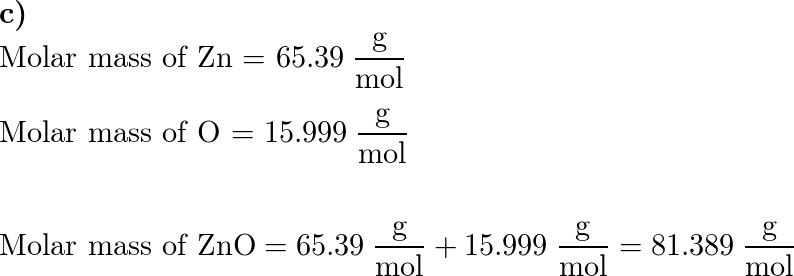40. Calculate the percentage of nitrogen in ammonium nitrate, (NH4NO3). (N = 14, H = 1, 0 = 16) - Brainly.in
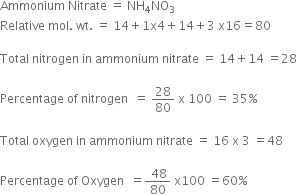
Calculate the percentage of nitrogen and oxygen in ammonium nitrate. (relative molecular mass of ammonium nitrate is 80, H=1, N=14, O=16.) - Zigya
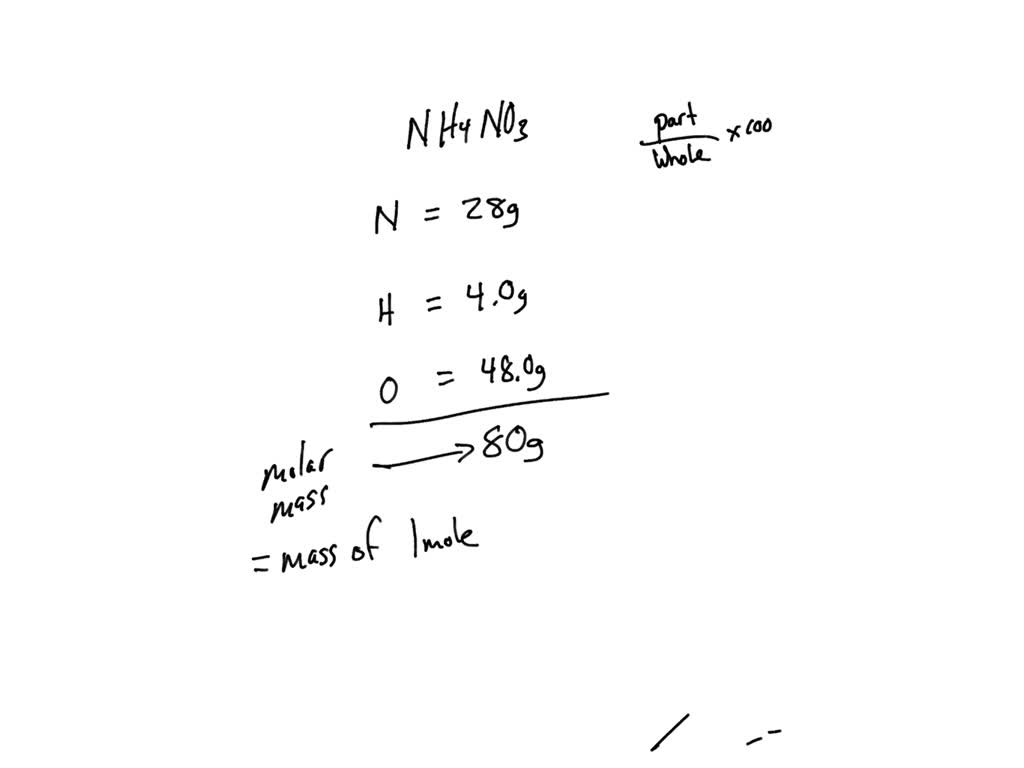
SOLVED: The last step is to calculate the percent by mass of each element in ammonium nitrate (NH4NO3). The masses of the elements in one mole of the compound are: mass N =

SOLVED: Ammonium nitrate, NH4 NO3, is used as a nitrogen fertilizer and in explosives. What is the molar mass of NHA NO3 ? | Numerade
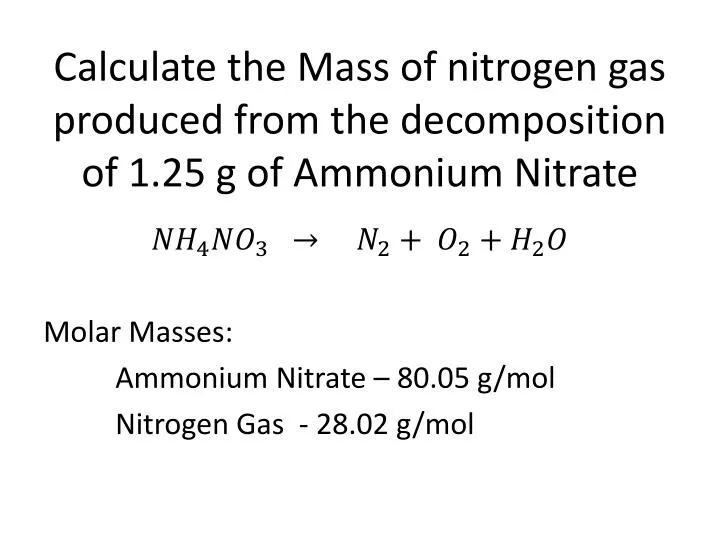
PPT - Calculate the Mass of nitrogen gas produced from the decomposition of 1.25 g of Ammonium Nitrate PowerPoint Presentation - ID:2338772

An aqueous solution is to be prepared that will be 7.51% by mass ammonium nitrate. What mass of NH_4NO_3 and what mass of water will be needed to prepare 1.25 kg of
![Determine the percentage of oxygen in Ammonium nitrate [Relative molecular mass of ammonium nitrate is 80, O = 16] - Chemistry - Analytical Chemistry Uses of Ammonium Hydroxide and Sodium Hydroxide - 14439229 | Meritnation.com Determine the percentage of oxygen in Ammonium nitrate [Relative molecular mass of ammonium nitrate is 80, O = 16] - Chemistry - Analytical Chemistry Uses of Ammonium Hydroxide and Sodium Hydroxide - 14439229 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_8dbf6cc00f3eb3e37314e6d8d70b3d39.png)
Determine the percentage of oxygen in Ammonium nitrate [Relative molecular mass of ammonium nitrate is 80, O = 16] - Chemistry - Analytical Chemistry Uses of Ammonium Hydroxide and Sodium Hydroxide - 14439229 | Meritnation.com

If the molecular mass of the compound 'X' is found to be 108 g/mol, then its molecular formula is:C_{12}H_{16}N_{4}C_{6}H_{6}N_{2}C_{9}H_{12}N_{3}C_{6}H_{8}N_{2}

OneClass: An aqueous solution of ammonium nitrate,NH4NO3 , contains 2.16 grams of ammonium nitrate an...
Calculate the percentage of nitrogen and oxygen in ammonium nitrate. [Relative molecular mass of ammonium nitrate is 80, H = 1, N = 14, O = 16] - Sarthaks eConnect | Largest Online Education Community

Fertilizers are added to the soil to improve crop yields. A farmer has a choice of two fertilizers, ammonium nitrate NH<sub>4</sub>NO<sub>3</sub> or diammonium hydrogen phosphate (NH<sub>4)2</sub>HPO<sub>4</sub>) (video) | Nitrogen and its Compounds

![Ammonium Nitrate [NH4NO3] Molecular Weight Calculation - Laboratory Notes Ammonium Nitrate [NH4NO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/ammonium-nitrate-molecular-weight-calculation-300x174.jpg)
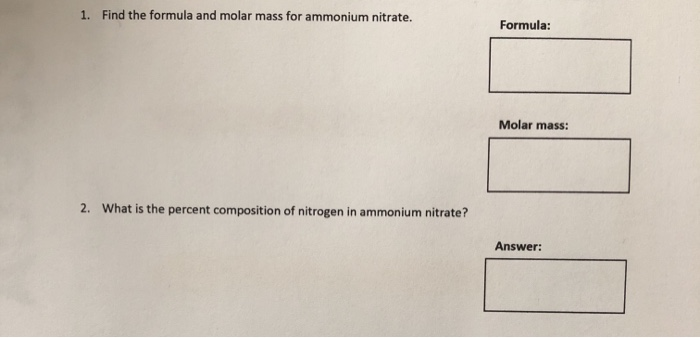



![N5 Chemistry] How do I approach this question? : r/chemistryhelp N5 Chemistry] How do I approach this question? : r/chemistryhelp](https://i.redd.it/s59m878jja9a1.jpg)


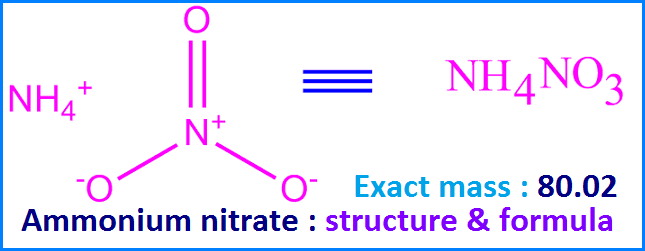
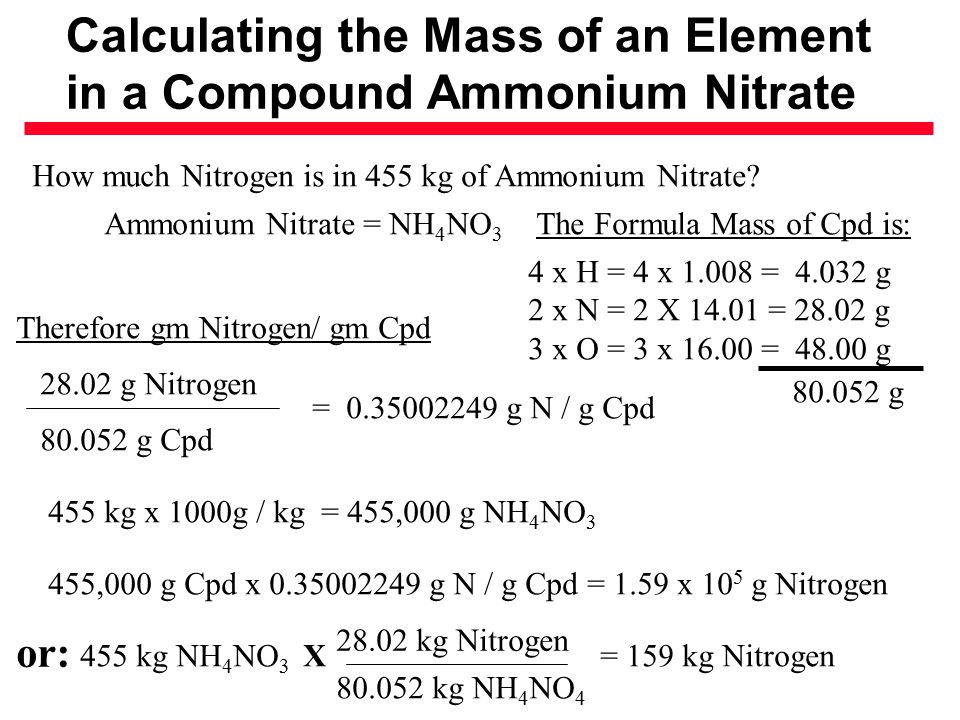
![Calculate the percentage of nitrogen in ammonium nitrate. [NH4NO3] [N= Calculate the percentage of nitrogen in ammonium nitrate. [NH4NO3] [N=](https://static.doubtnut.com/ss/web/6794208.webp)