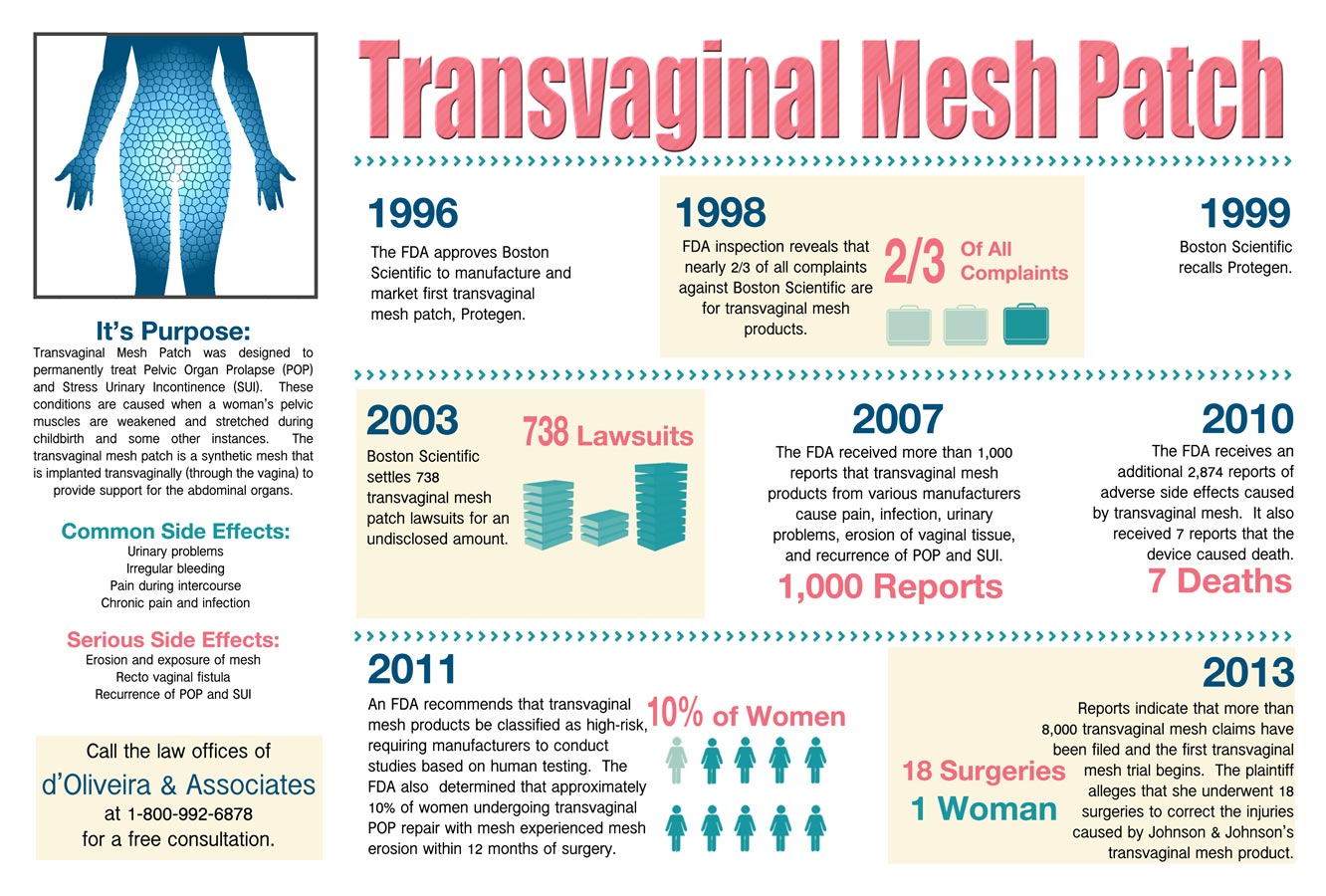
Crystal Mesh: How Addiction to Money Turned Medical Device Makers, the FDA, and Doctors Into Street Dealers: Mundy, Alicia, Banmiller, Jennifer: 9781733431507: Amazon.com: Books

ICS 2019 Abstract #693 The impact of the 2011 U.S. Food and Drug Administration transvaginal mesh communication on utilization of synthetic midurethral sling procedures for stress urinary incontinence
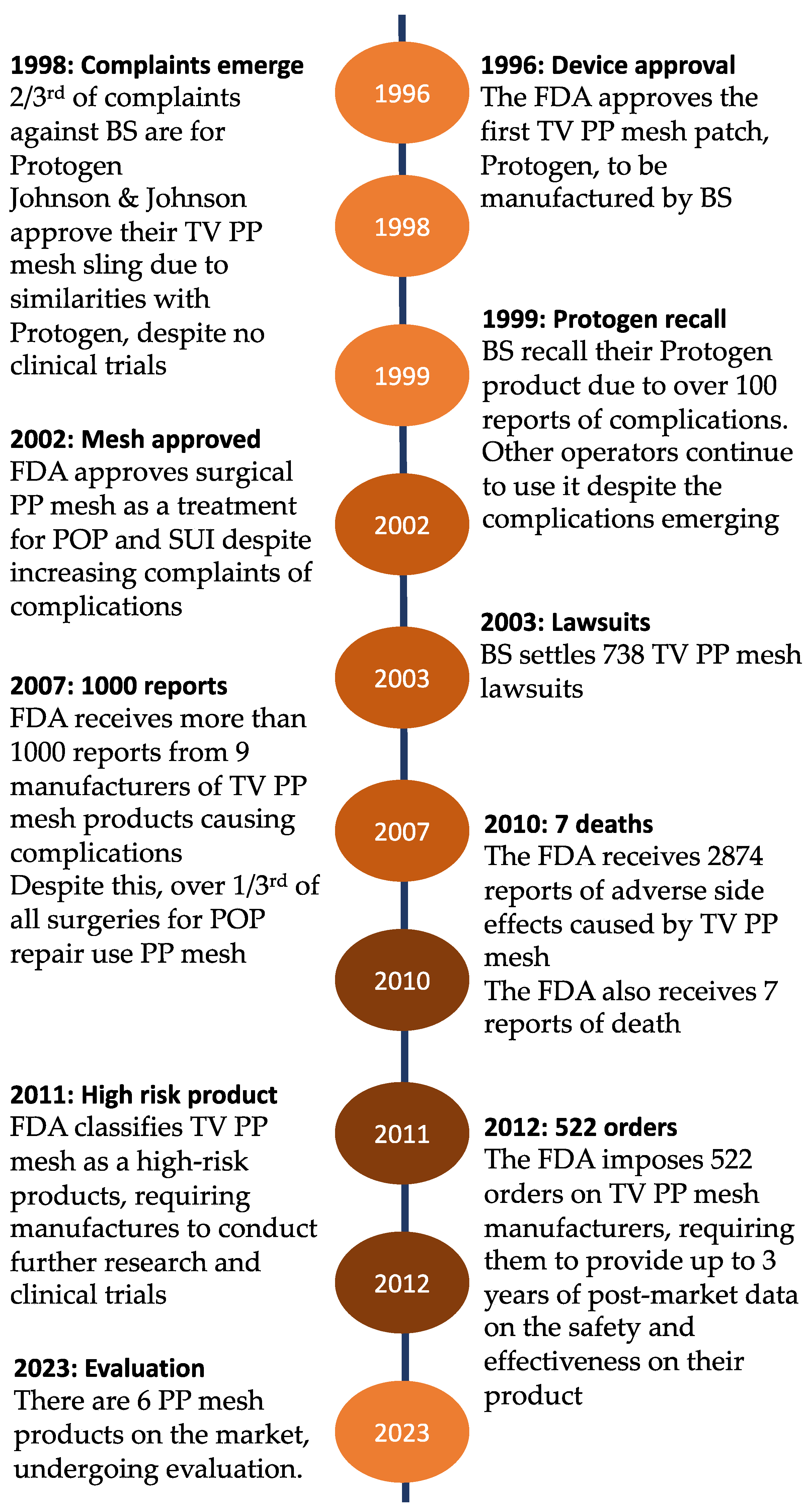
Biomedicines | Free Full-Text | Polypropylene Pelvic Mesh: What Went Wrong and What Will Be of the Future?