September 15, 2022 Ulike Co., Ltd ℅ Sun Cindy Senior Consultant PureVision Ai, Inc. 111 Town Square Place, Suite 1203 New Jers

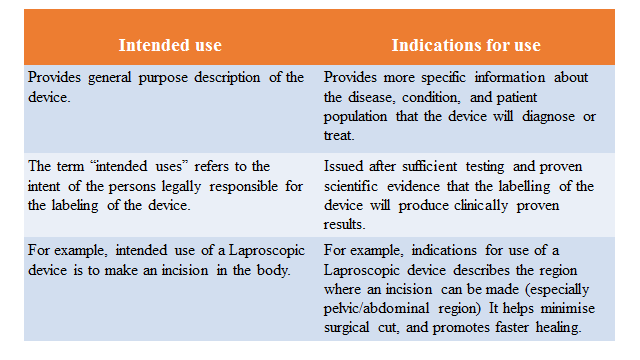
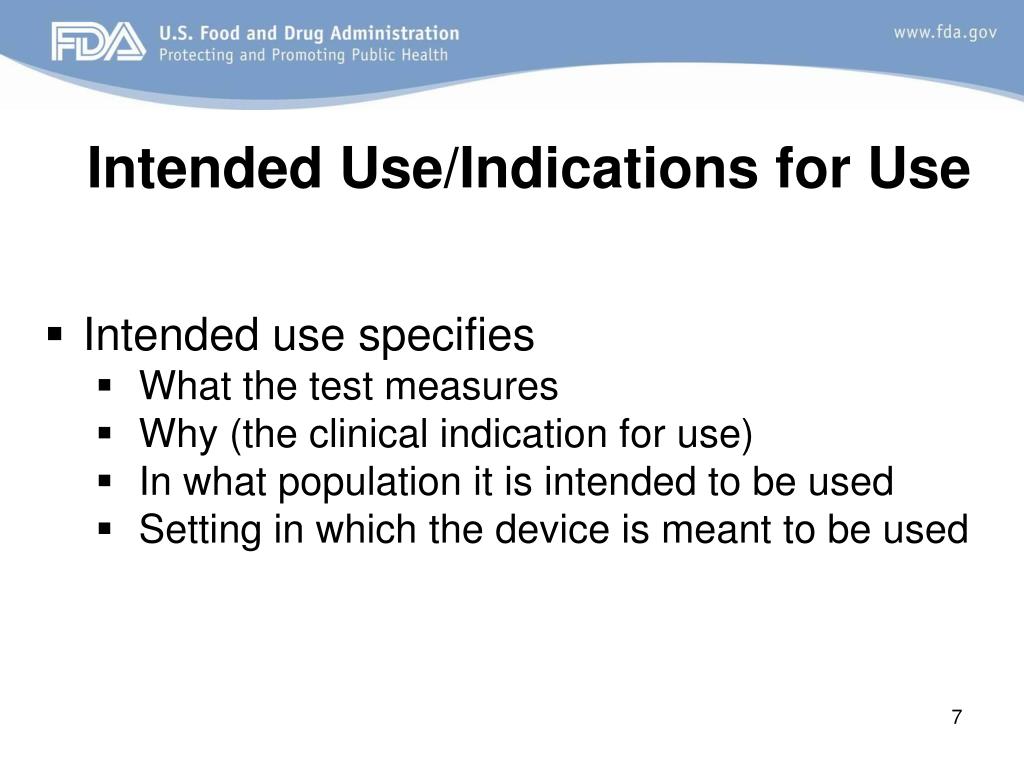
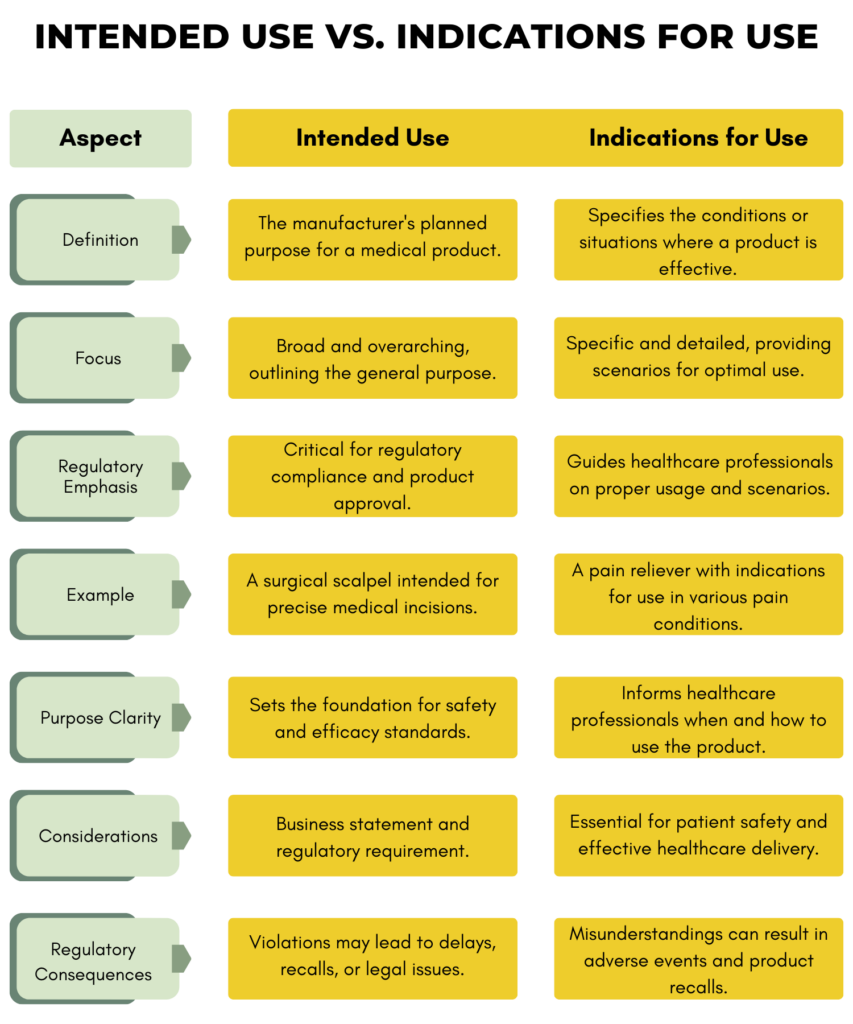
The Difference Between Intended Use and Indications of Use (And Why These Statements Are So Important)

The 510(k) Program Roy Baby, Investigator US Food & Drug Administration 4040 N Central Expressway, Dallas, TX ppt video online download

FDA's role in the innovation and evaluation of evolving computer-aided diagnosis (CAD) solutions Kyle J. Myers, Ph.D. Director, Division of Imaging, Diagnostics, - ppt download
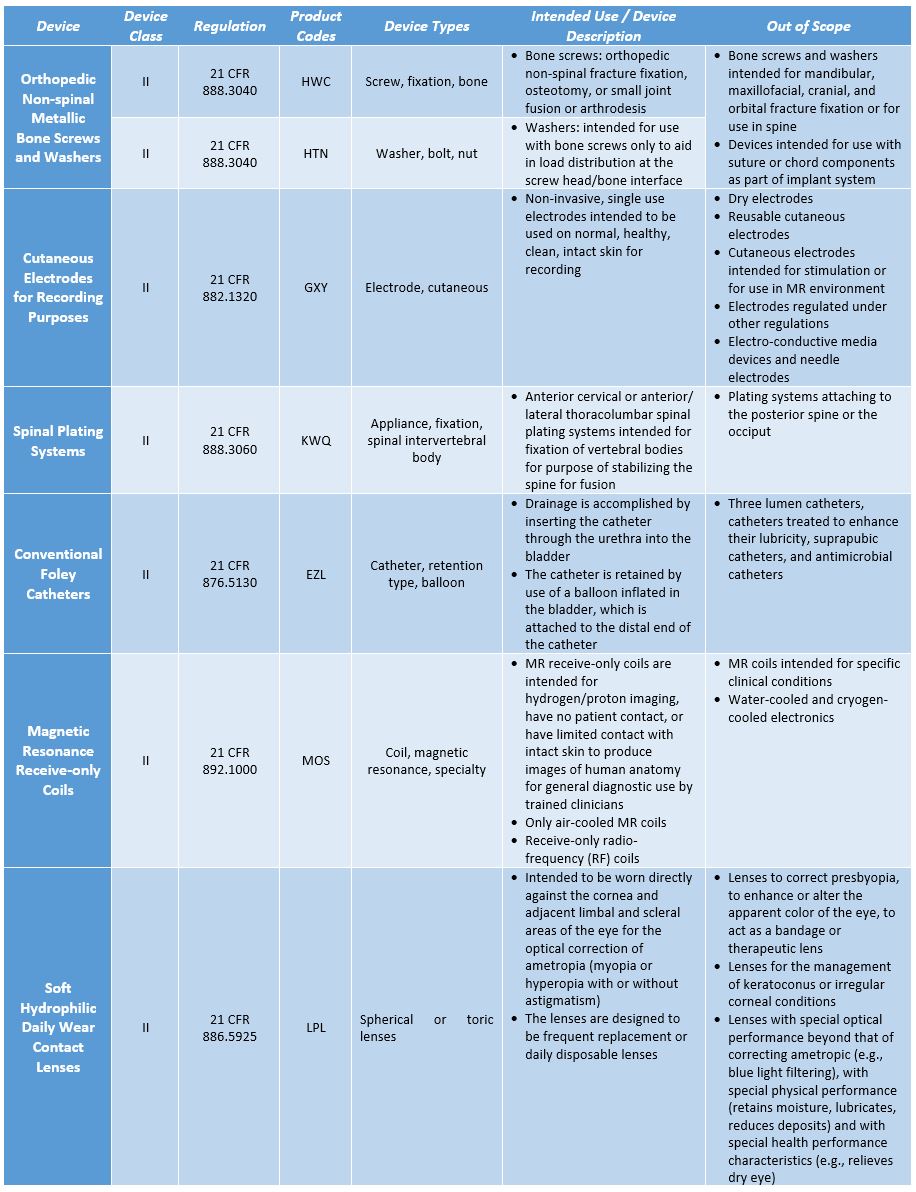
FDA's Safety And Performance-based Pathway An Alternative To Substantial Equivalence For 510(k) Submissions
%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png?width=4250&name=(cover)%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png)
The Difference Between Intended Use and Indications of Use (And Why These Statements Are So Important)