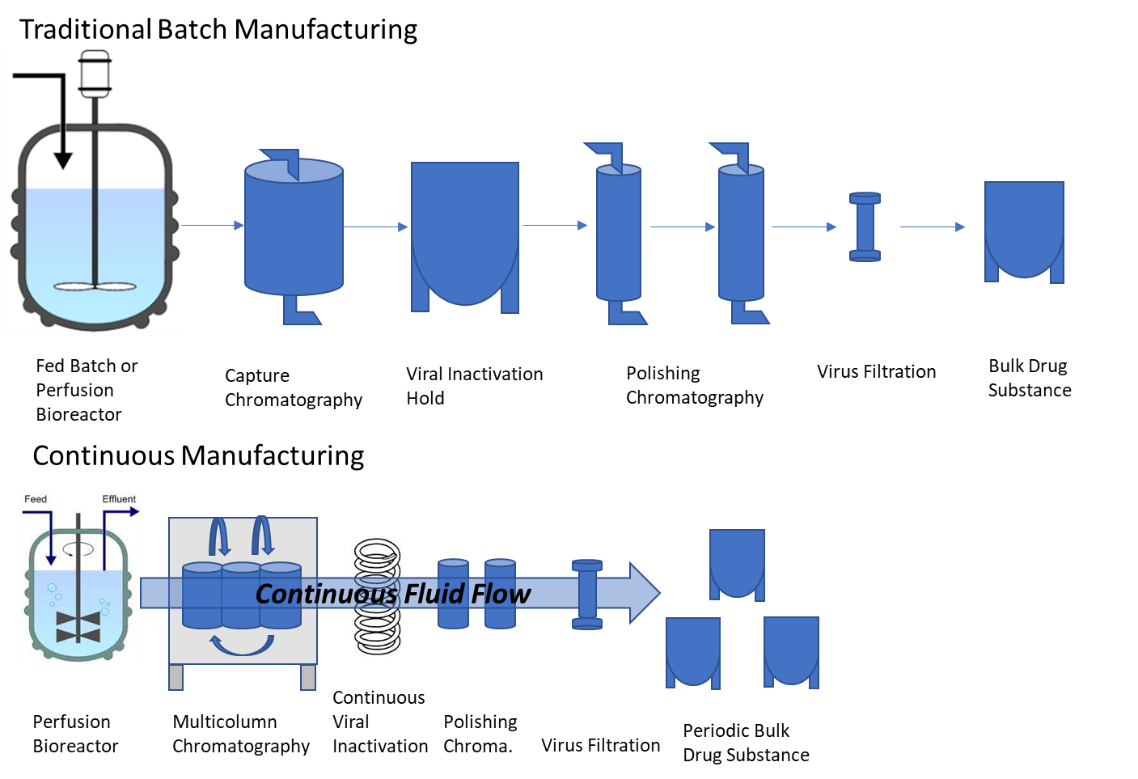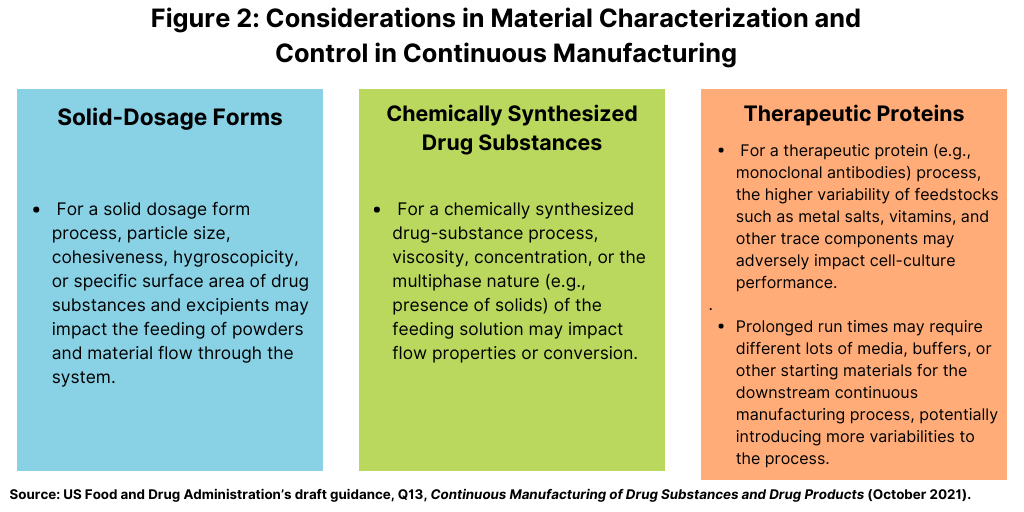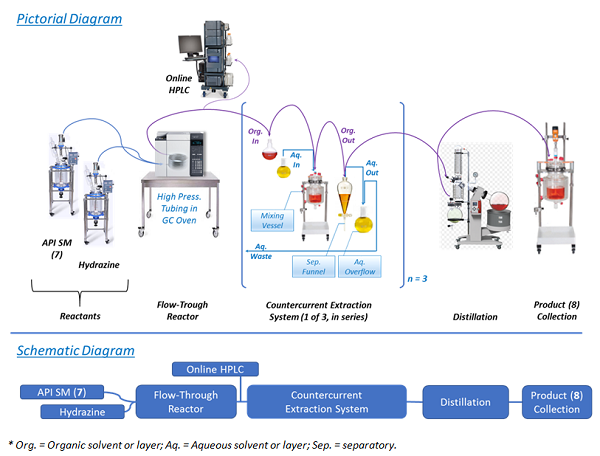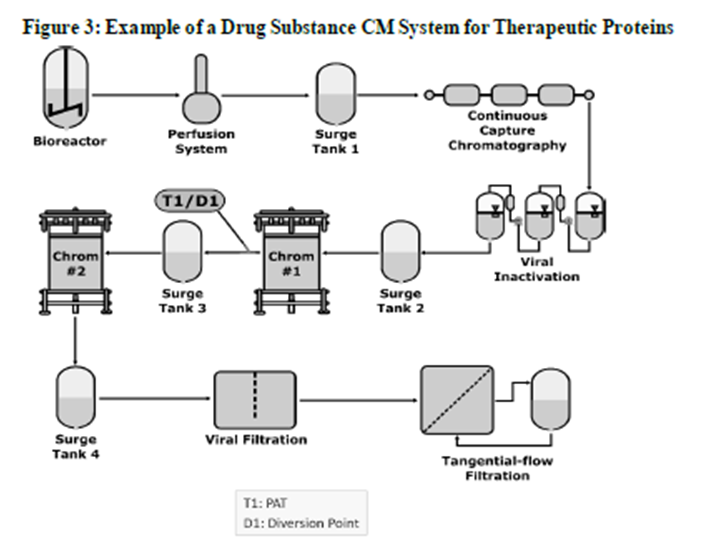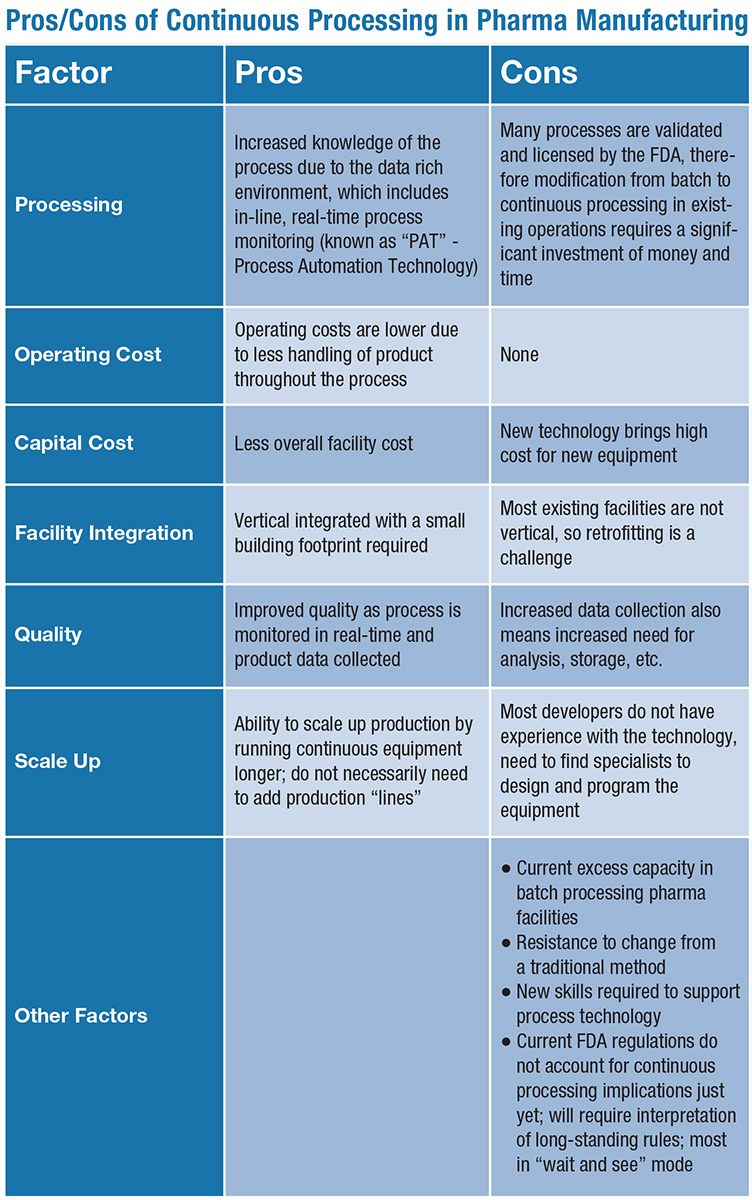
How Continuous Processing is Impacting U.S. Drug Manufacturing Facilities | Trade and Industry Development
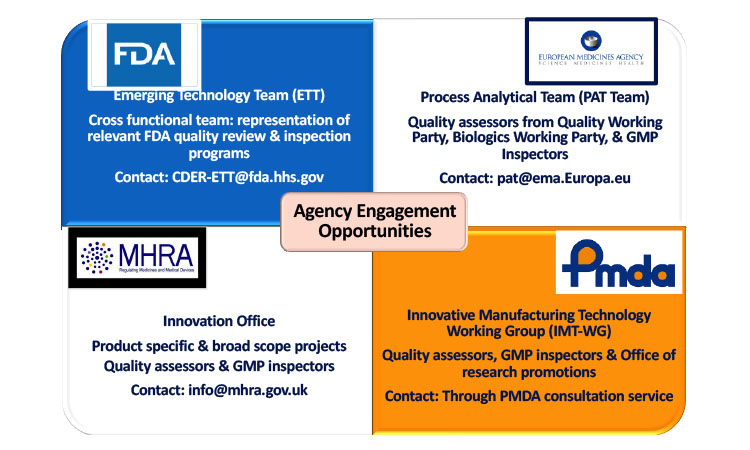
Regulatory Progress - Global Advancement of Continuous Manufacturing for Pharma | Pharmaceutical Engineering
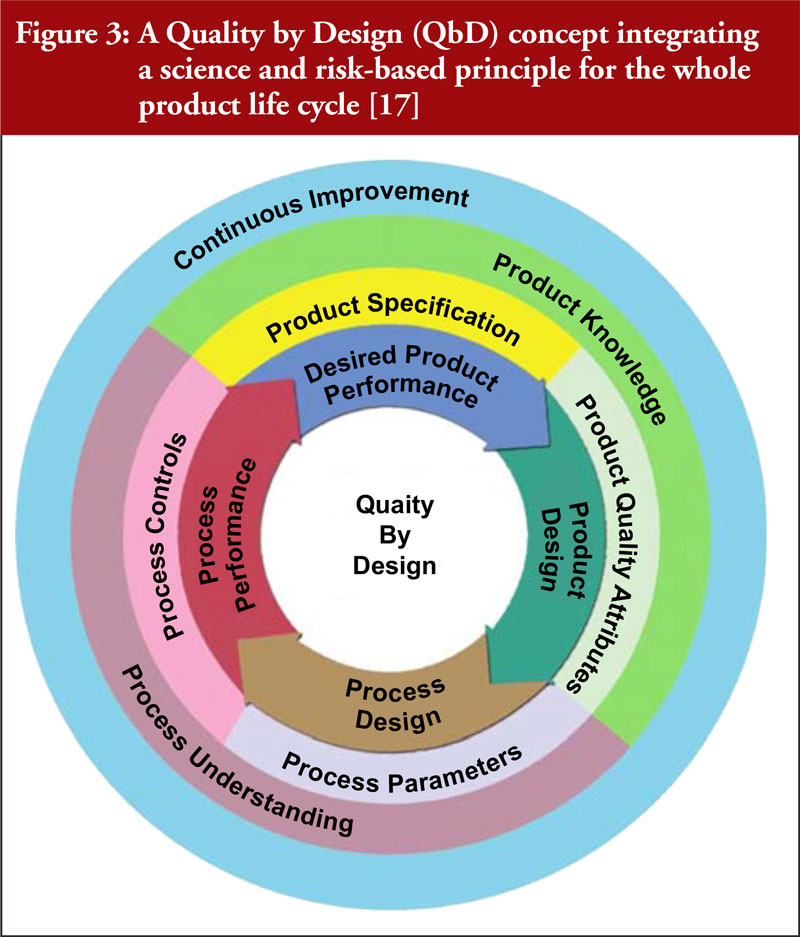
Continuous manufacturing versus batch manufacturing: benefits, opportunities and challenges for manufacturers and regulators - GaBI Journal

From Data Integrity to Continuous Manufacturing: A Review of Withdrawn and Revised FDA Guidelines in the

FDA Issues ICH Final Guidance for Continuous Manufacturing (CM) and Artificial Intelligence (AI) in Drug Manufacturing – ISOMETRIC CONSULTING

Continuous Manufacturing of Drug Substances and Drug Products - FDA announces guidance for Industry - Pharma Excipients

Approved drug products manufactured by continuous manufacturing (CM)... | Download Scientific Diagram