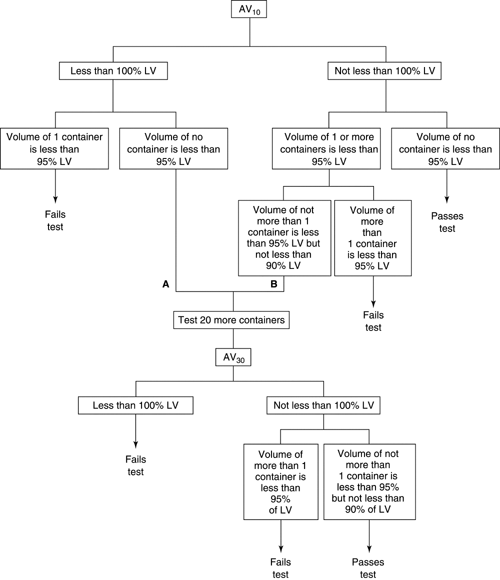
A Regulatory Perspective on Manufacturing Processes Pertaining to Lyophilized Injectable Products | The AAPS Journal
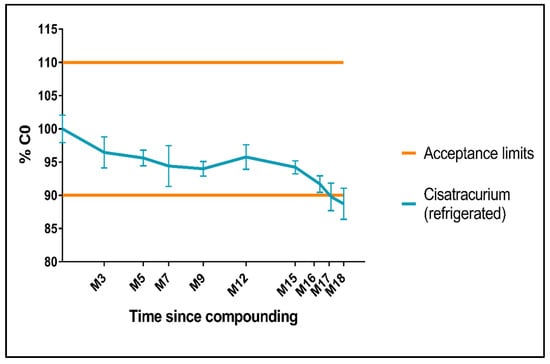
Pharmaceutics | Free Full-Text | Cistracurium Besylate 10 mg/mL Solution Compounded in a Hospital Pharmacy to Prevent Drug Shortages: A Stability Study Involving Four Degradation Products

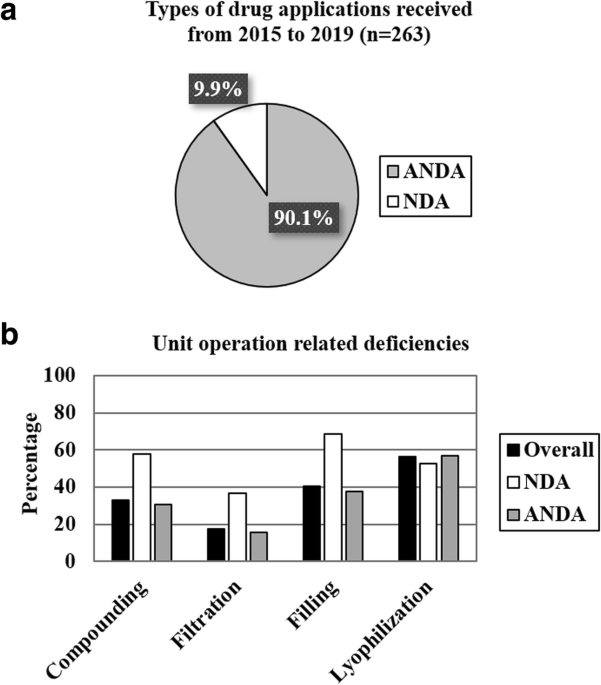
Closed System Transfer Devices (CSTDs): Understanding Potential Over- and Under- Dosing of Liquid Vial Drug Products and How to Generally Mitigate - ScienceDirect