EudraLex - Volume 10 Clinical trials guidelines and the impact of the new coming Regulation 536/2014

Eudralex Volume 4 Annex 1 – Room for Improvement? - Media Center - AUSTAR Connecting Extraordinary Ideas

EU cGMPs for ATMP - 2018: Guidelines on Good Manufacturing Practice Specific to Advanced Therapy Medicinal Products

PPT - REVISION OF EUDRALEX VOL. 4 - GMP Luisa Stoppa, Ph.D. Inspection and Certification Department Italian Medicines Agency PowerPoint Presentation - ID:711040
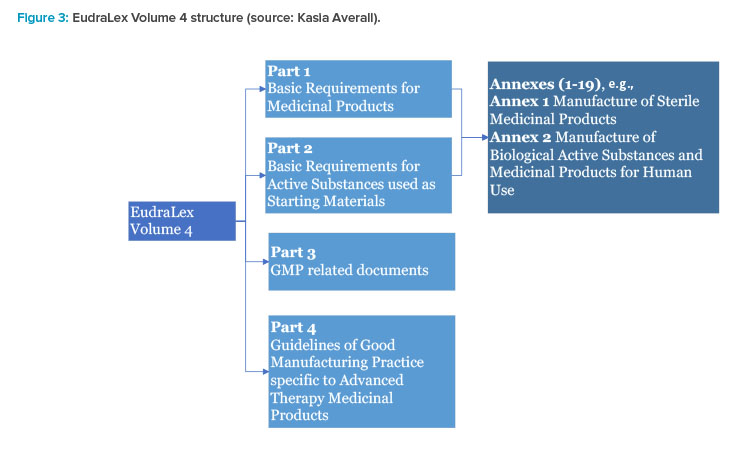
EUROPEAN COMMISSION Brussels, 03 February 2010 EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 Go

The Handbook of Basic GMP Requirements: Collected guidelines from Eudralex Volume 4, Part I “Basic Requirements for Medicinal Products”

Details for: Good manufacturing practices (GMP) guidelines: the rules governing medicinal products in the European Union; EudraLex volume 4 concise references/ › TUS Midlands Library catalog
Good Manufacturing Practice GMP Guidelines Eudralex Volume 4 : Mr. Rajesh. L. Dumpala | Mrs. Lakshmi Prasuna. R. Dumpala : Free Download, Borrow, and Streaming : Internet Archive

The Handbook of Basic GMP Requirements: Collected guidelines from Eudralex Volume 4, Part I “Basic Requirements for Medicinal Products”