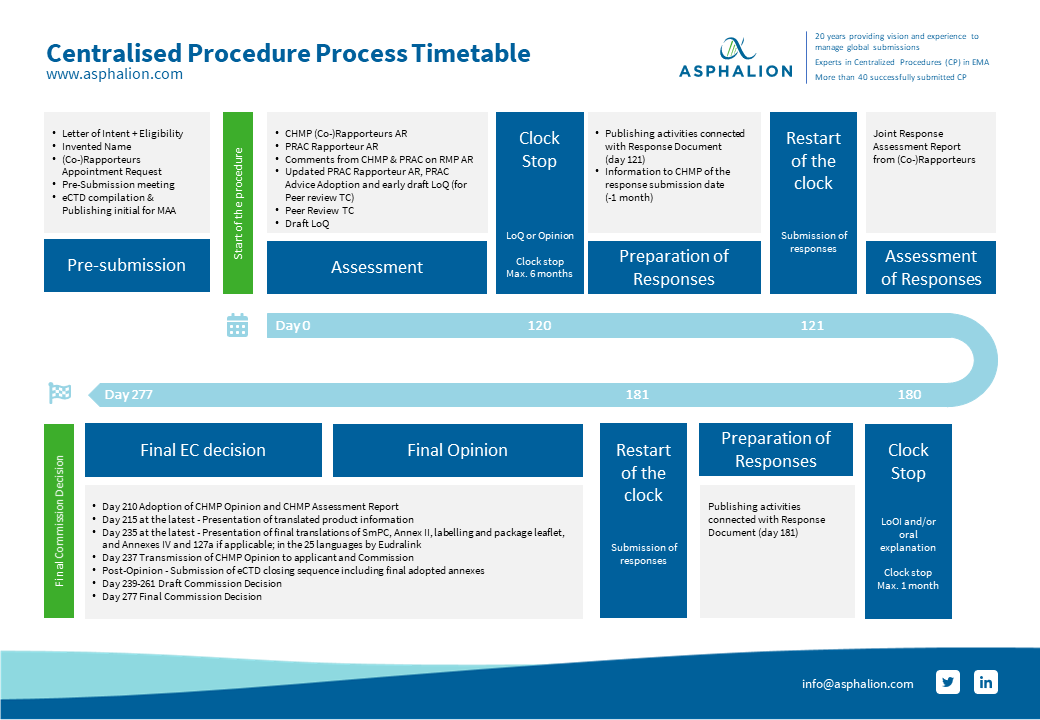
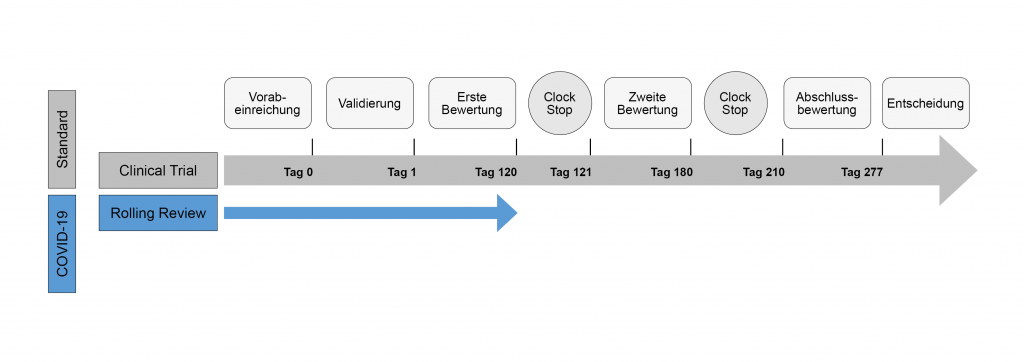
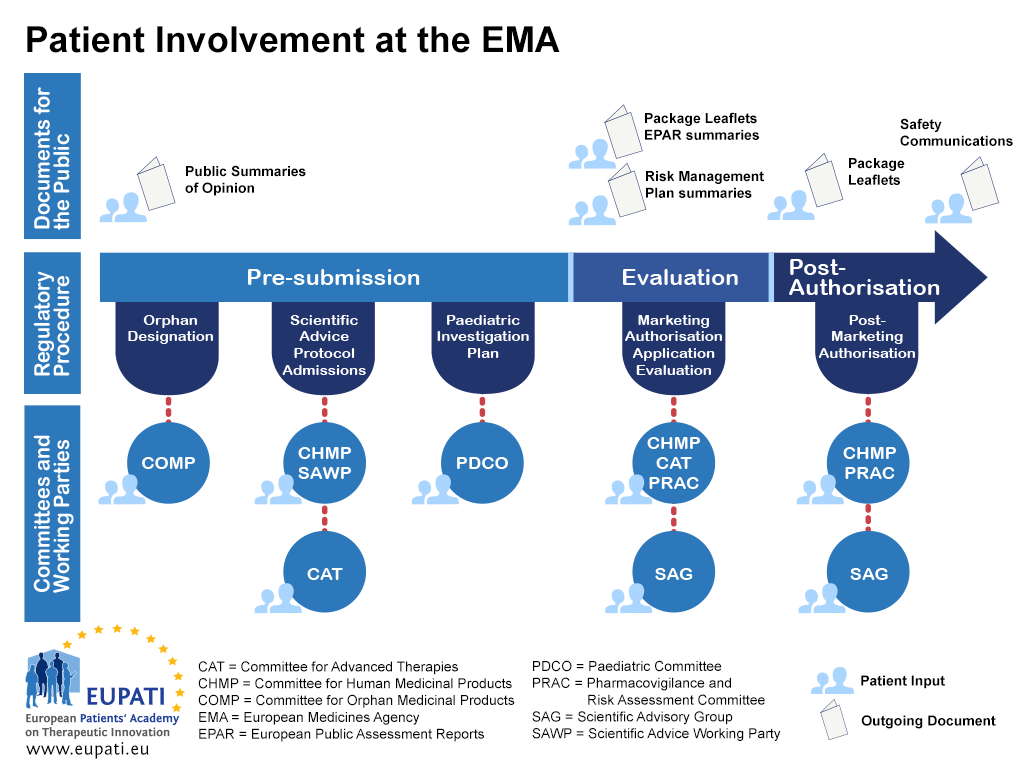
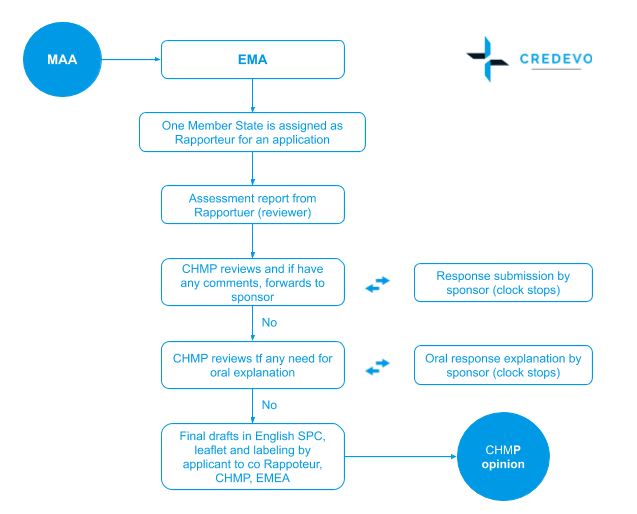
Review of MAAs according to the Centralised Procedure The evaluation of... | Download Scientific Diagram

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation. - Abstract - Europe PMC

Mutual recognition in the European system: A blueprint for increasing access to medicines? - ScienceDirect
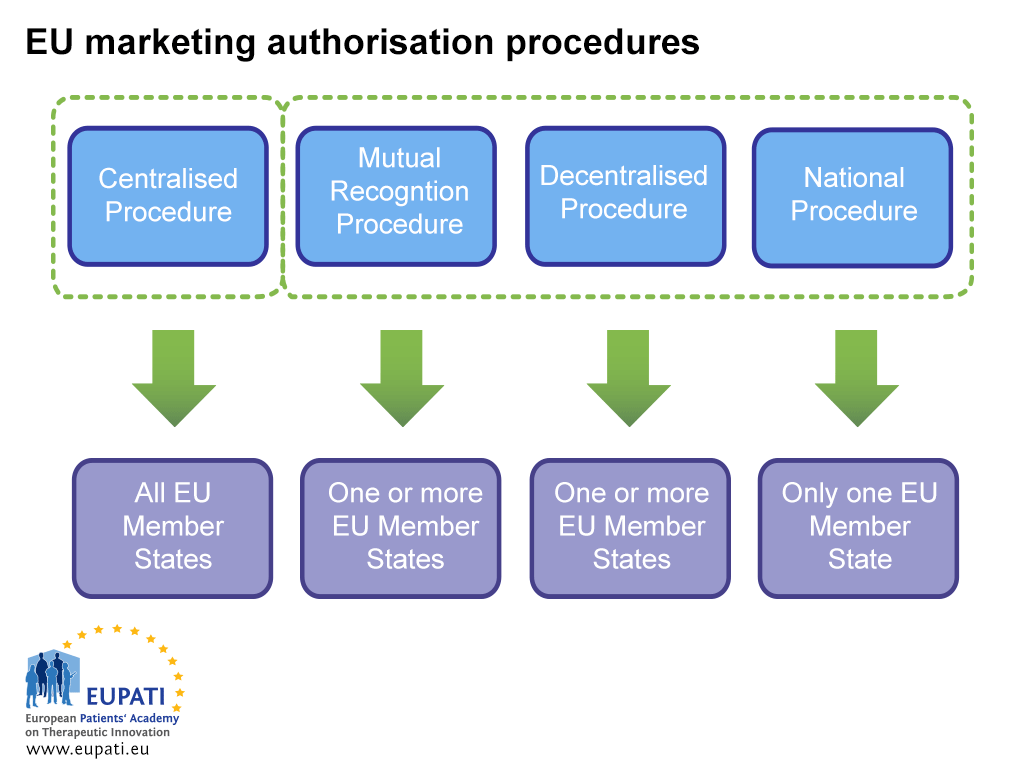
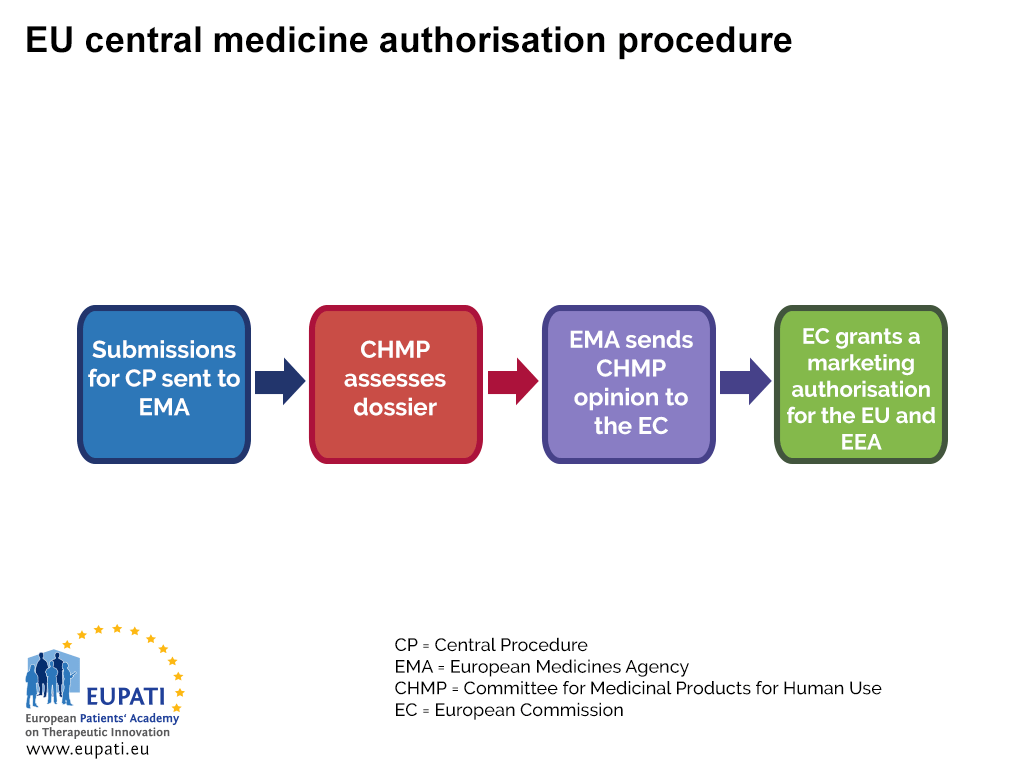
5. EU Regulatory procedures for a marketing authorisation (MA): Centralised procedure | EUPATI Open Classroom

Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy