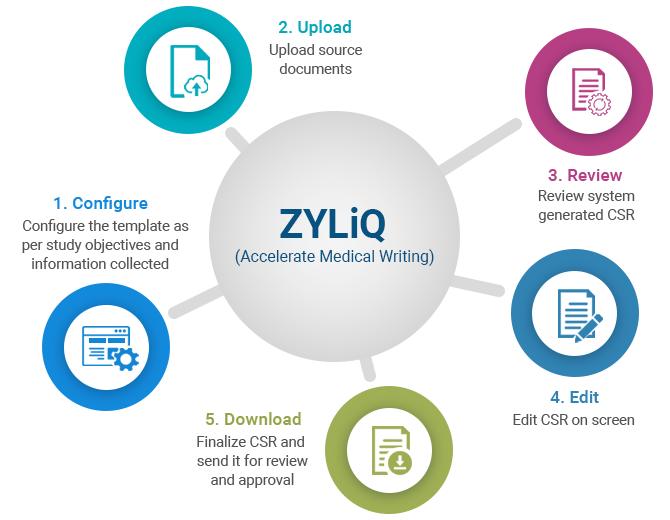
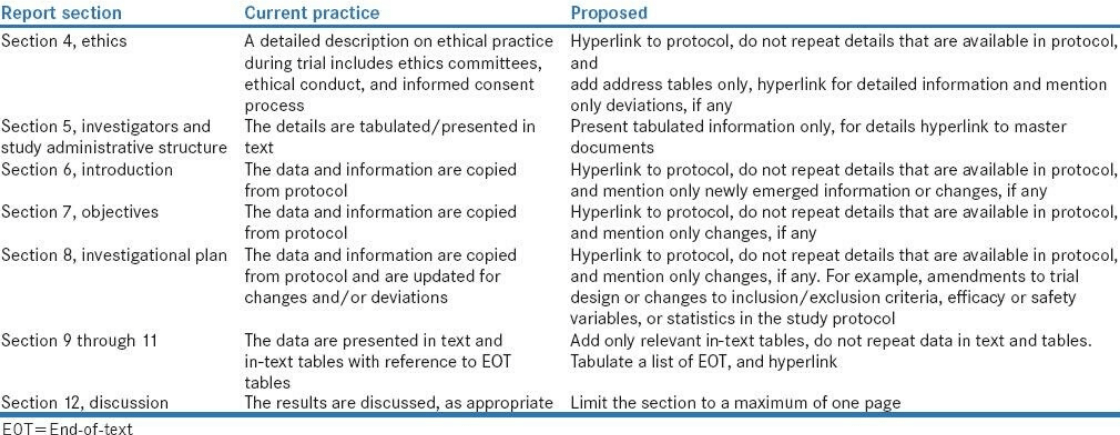
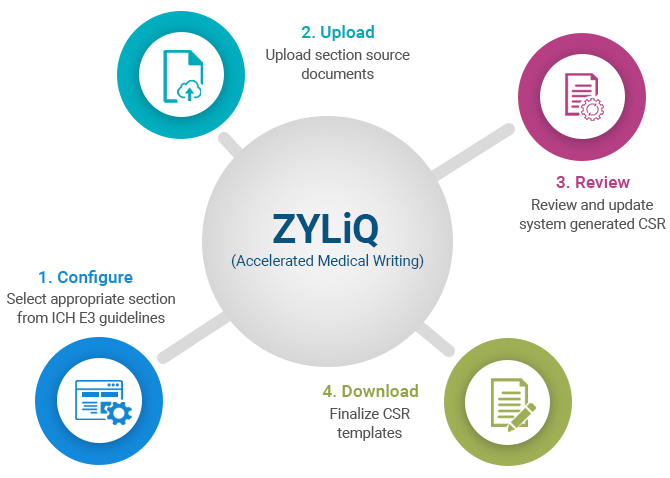
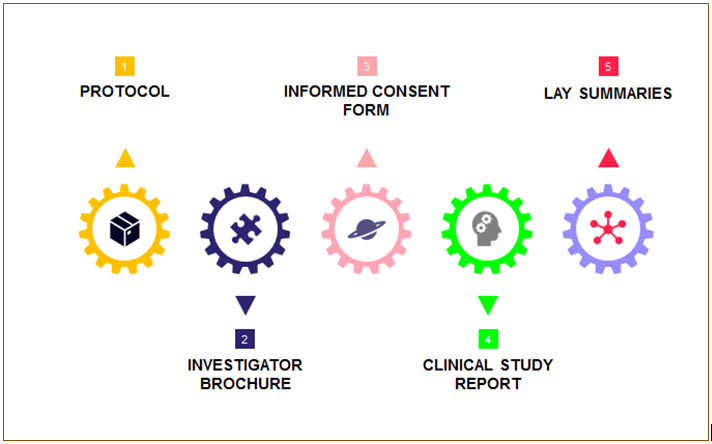
Medical writing for regulatory submission in clinical research and its challenges pdf2 by Medical Writing Experts - Issuu

Recommended procedure for medical writers working together with the... | Download Scientific Diagram

Clinical Study Report (CSR) - Report Level Publishing in ICH e3 | Freyr - Global Regulatory Solutions and Services Company