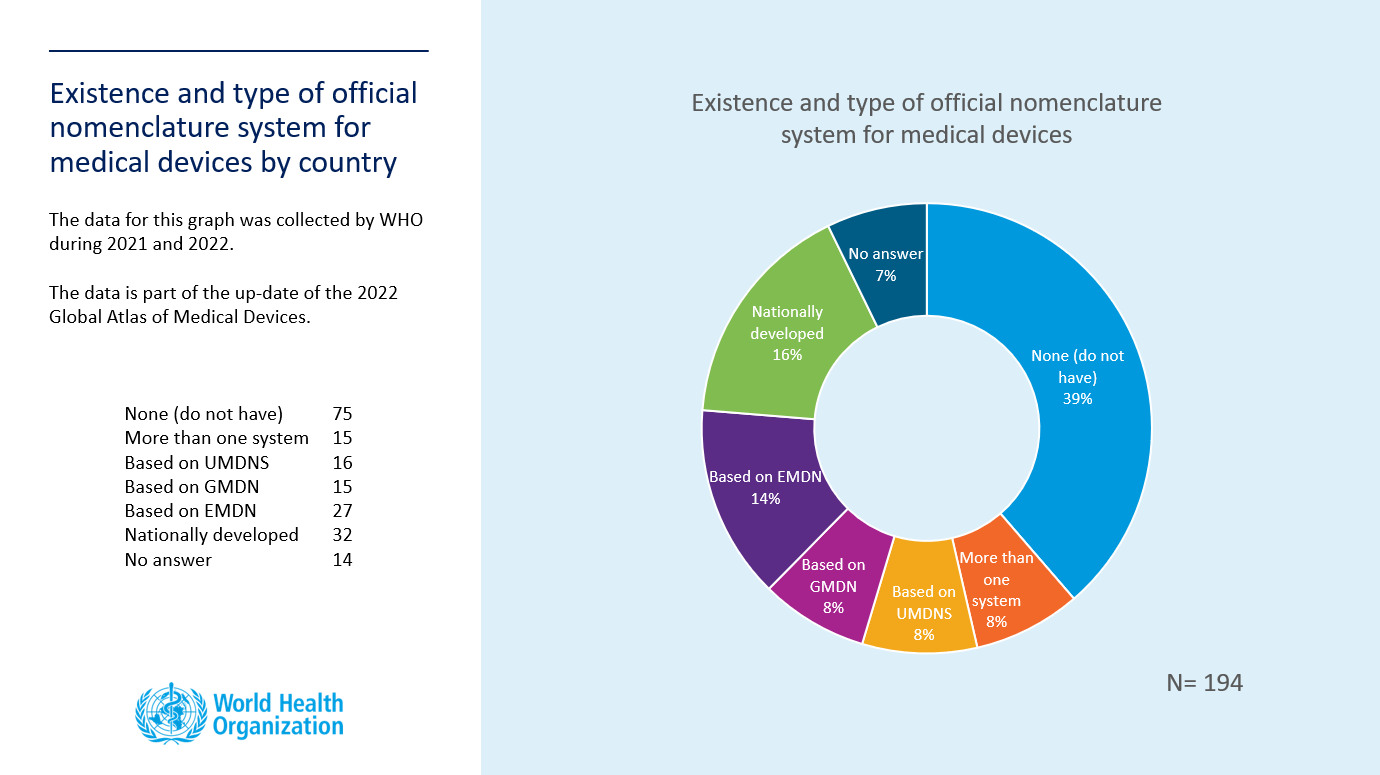
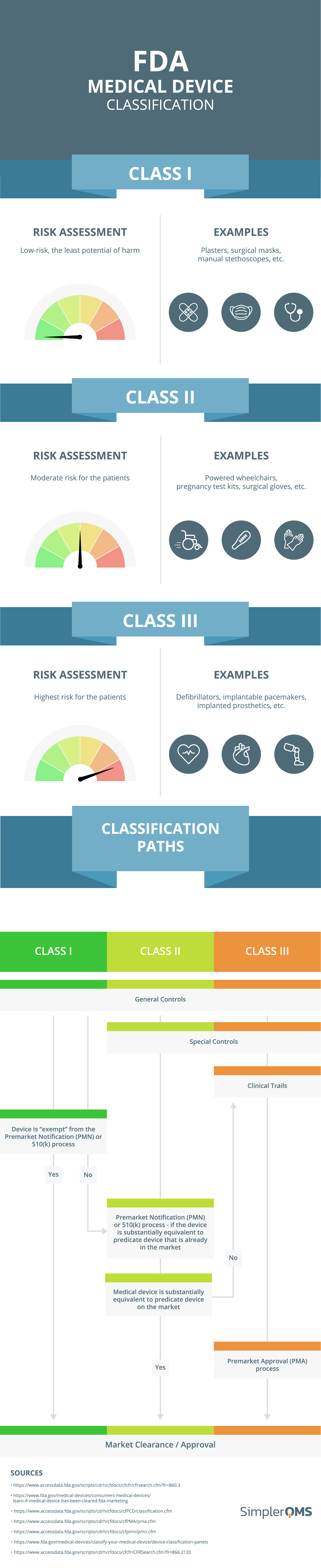
General classification and application types of medical devices for... | Download Scientific Diagram

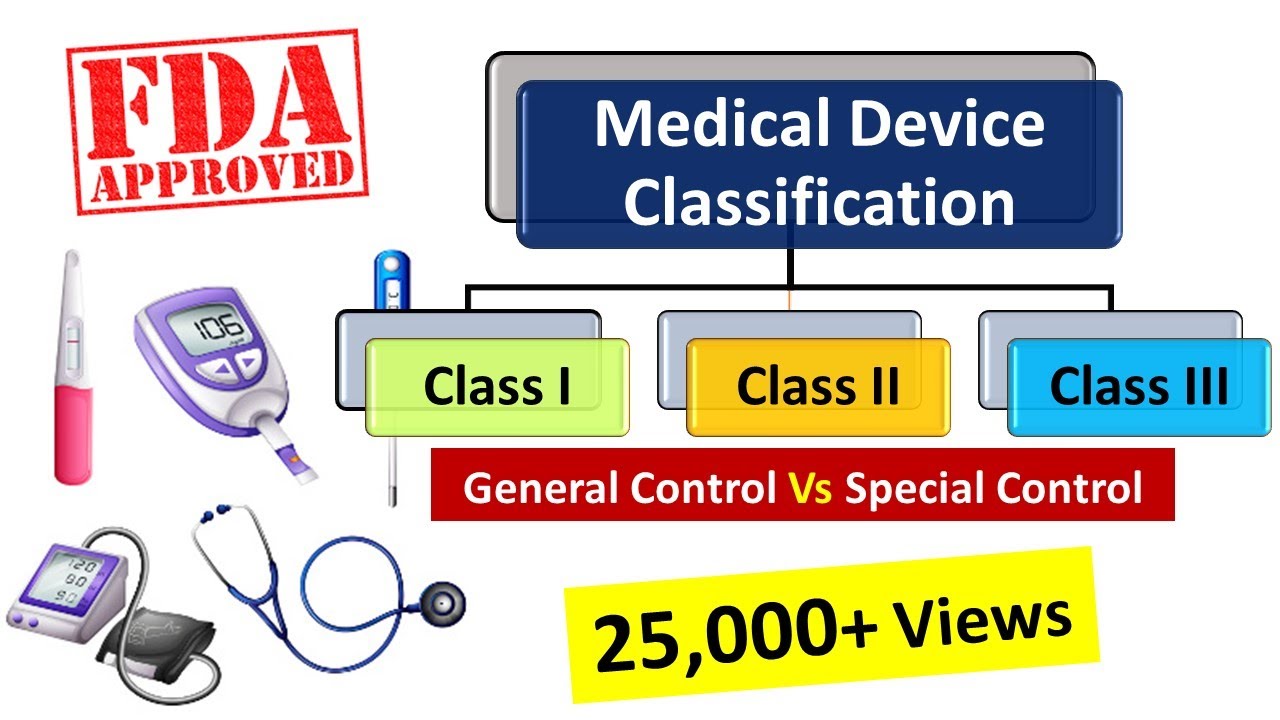
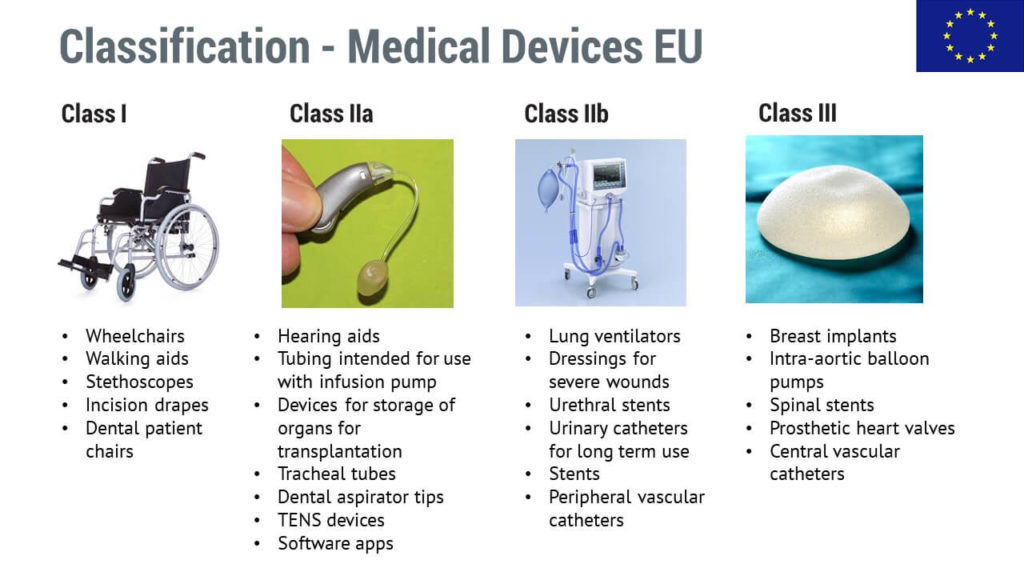
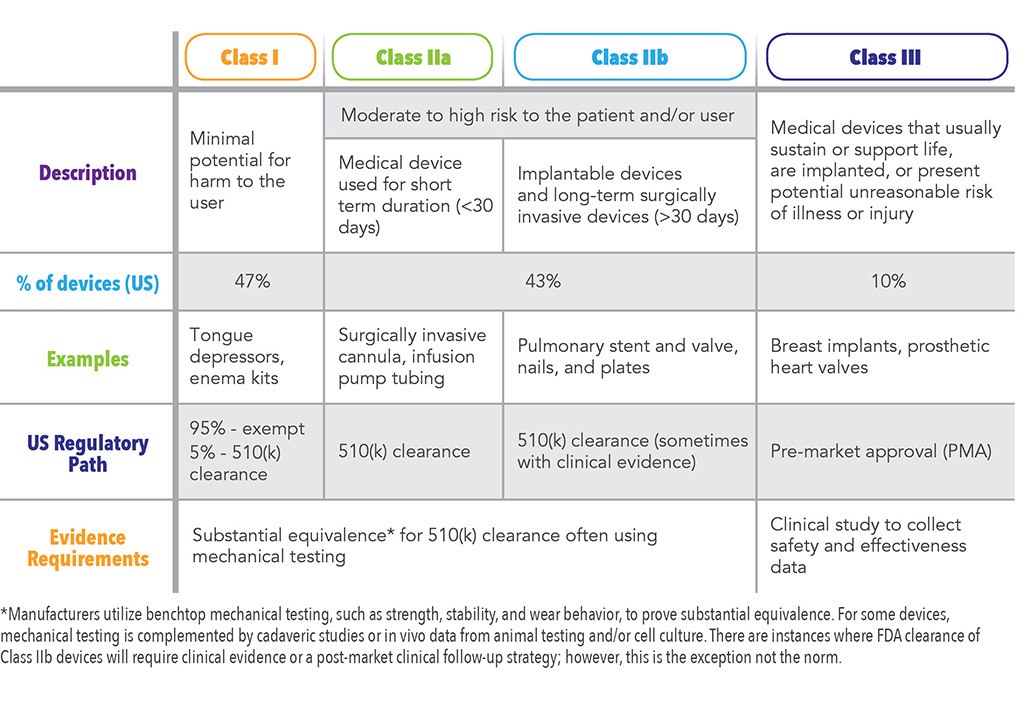
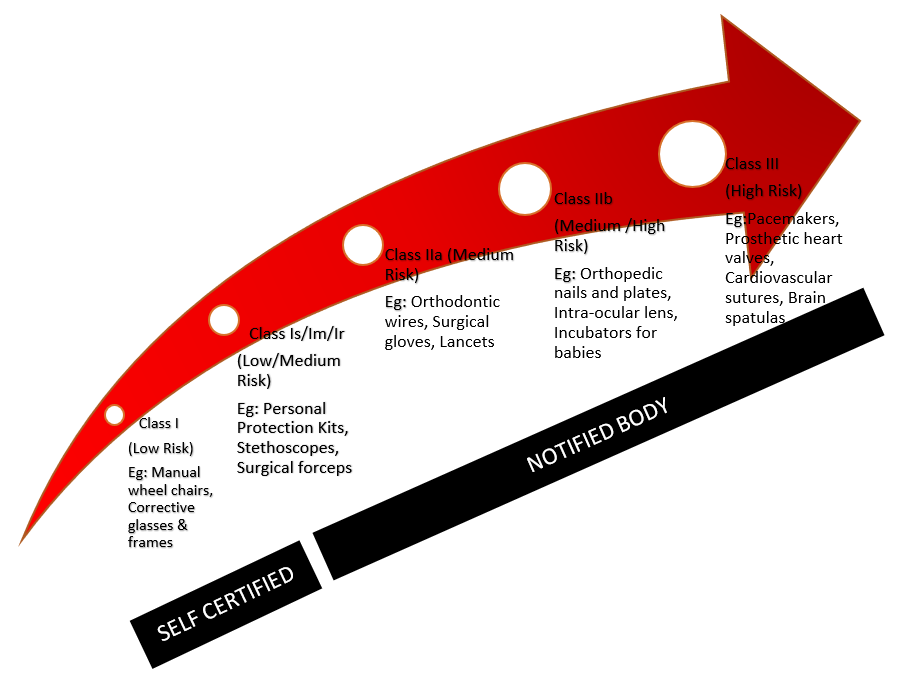
Medical devices are classified into three categories based upon risk to... | Download Scientific Diagram
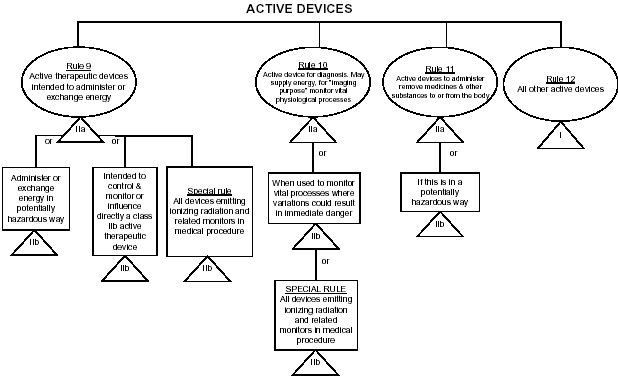
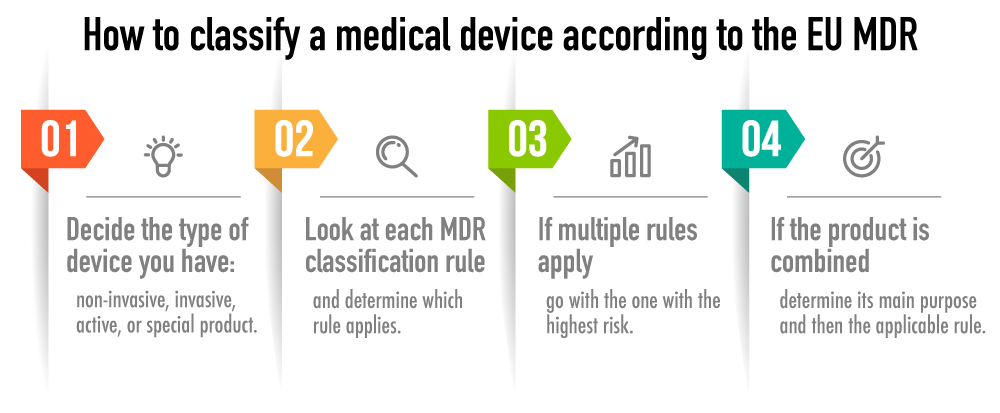
Guidelines for Classification of Medical Devices - CE Marking (CE Mark) for Medical Devices - EU Council Directive 93/42/EEC
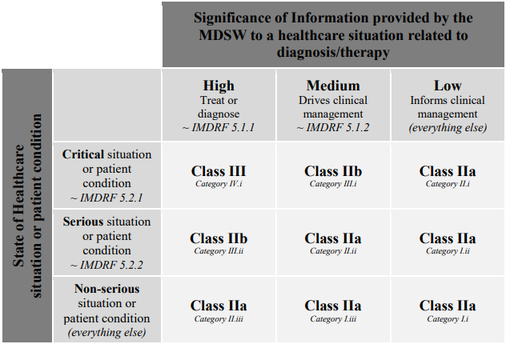
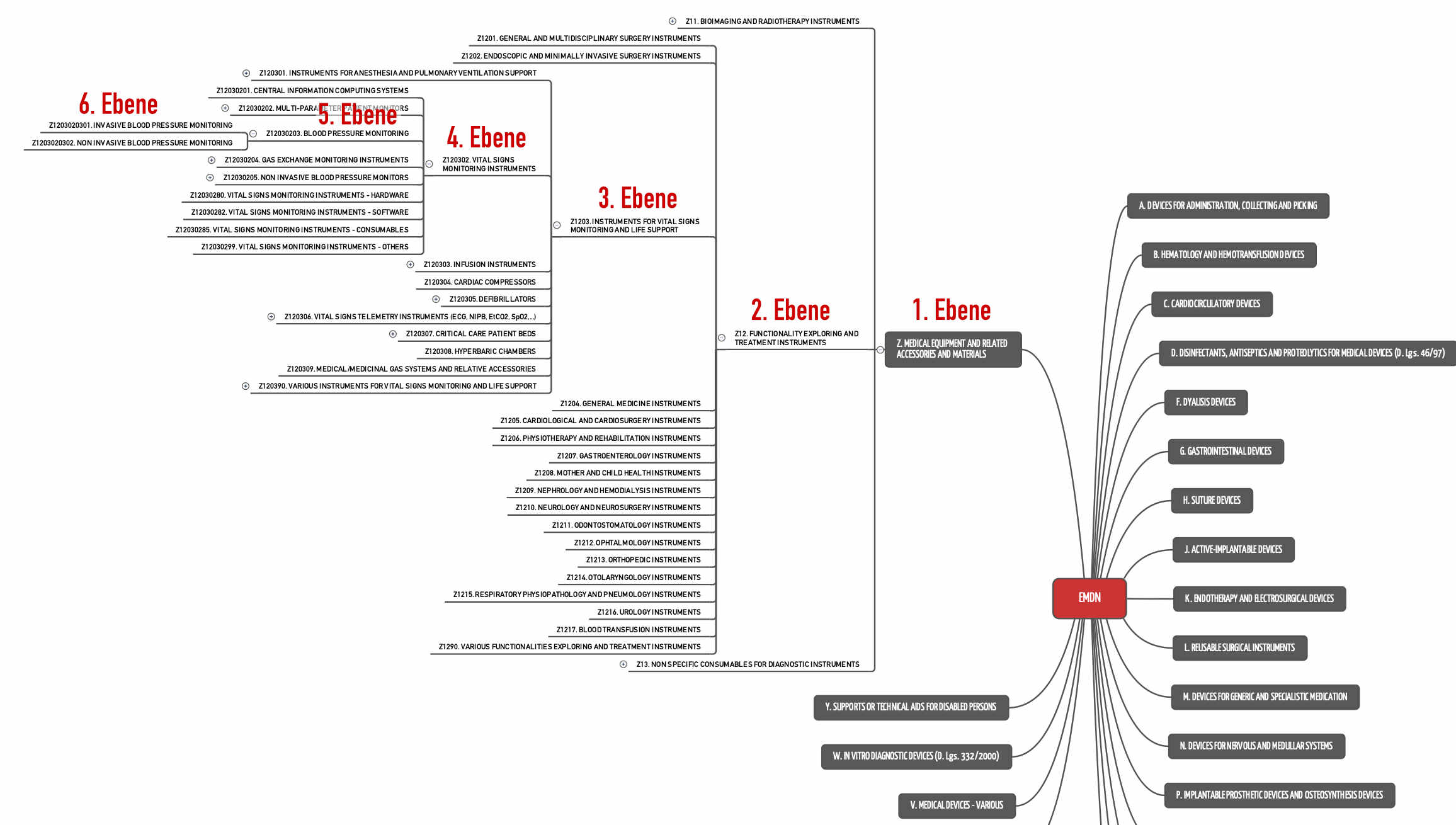
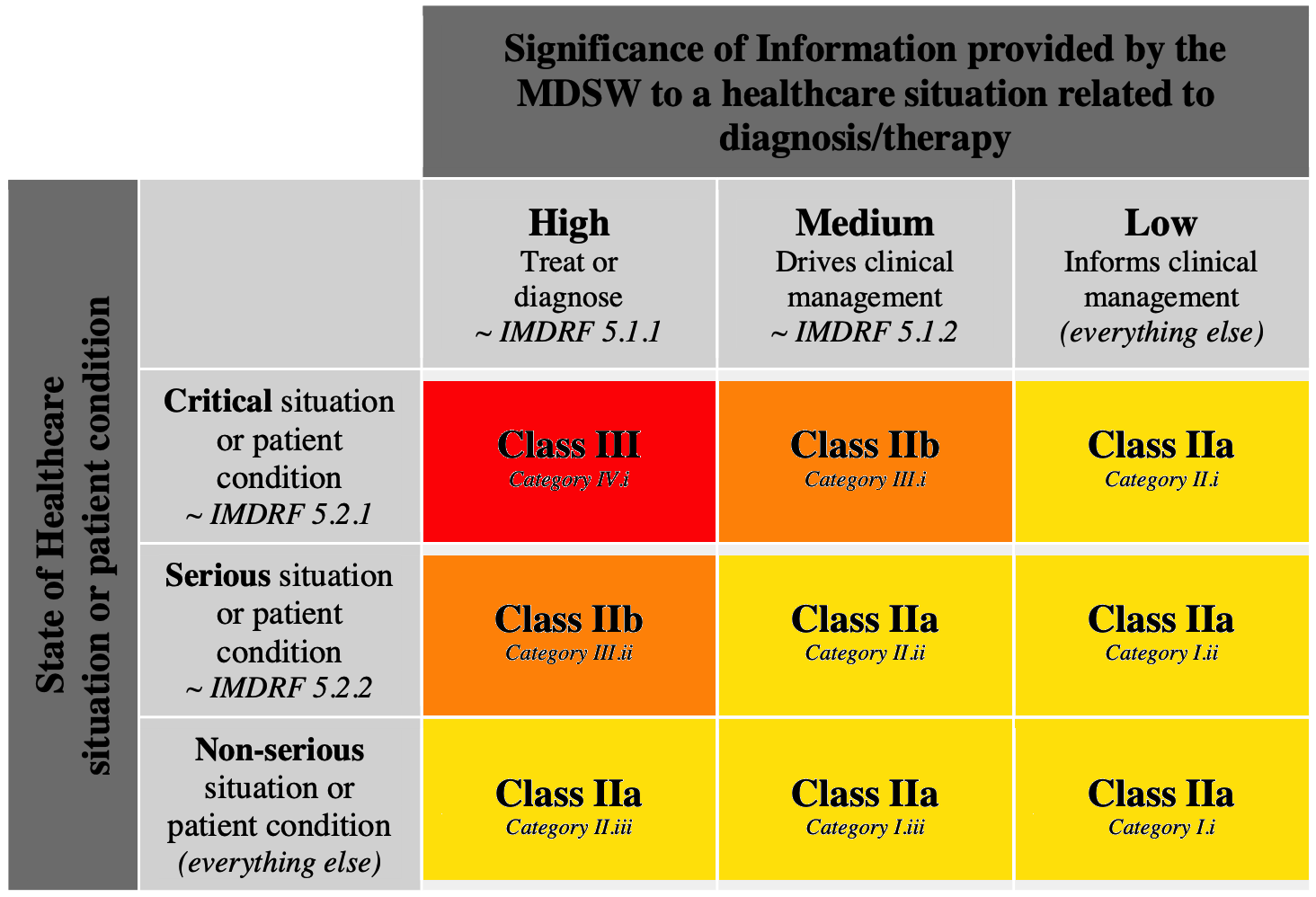
Software as a Medical Device in Europe – New Regulatory Regime About to Enter into Force – (Part 3 of 6) | MoFo Life Sciences