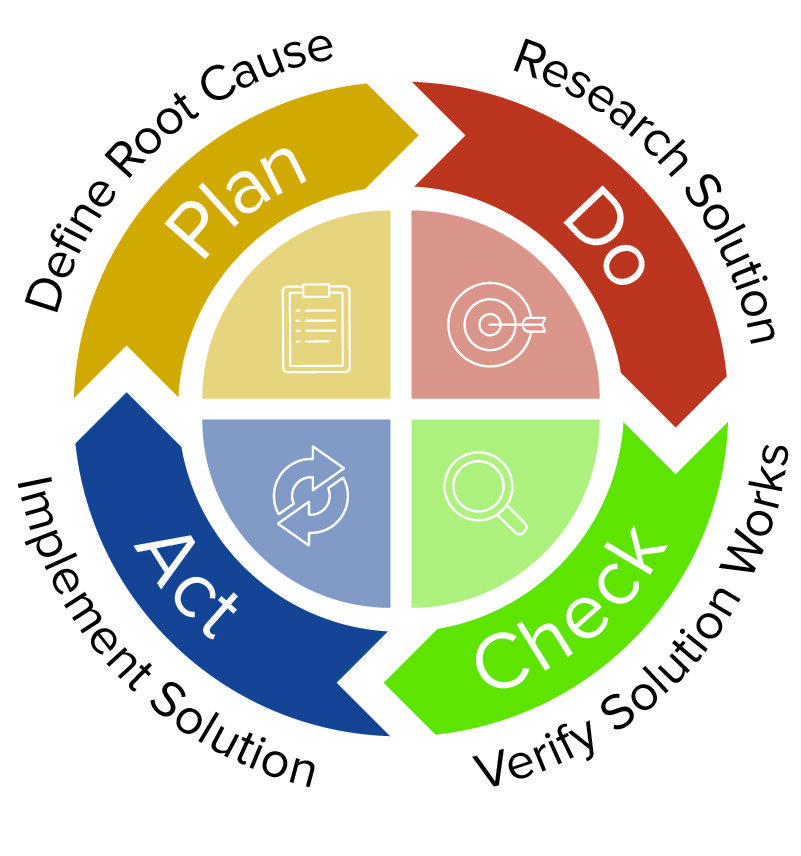
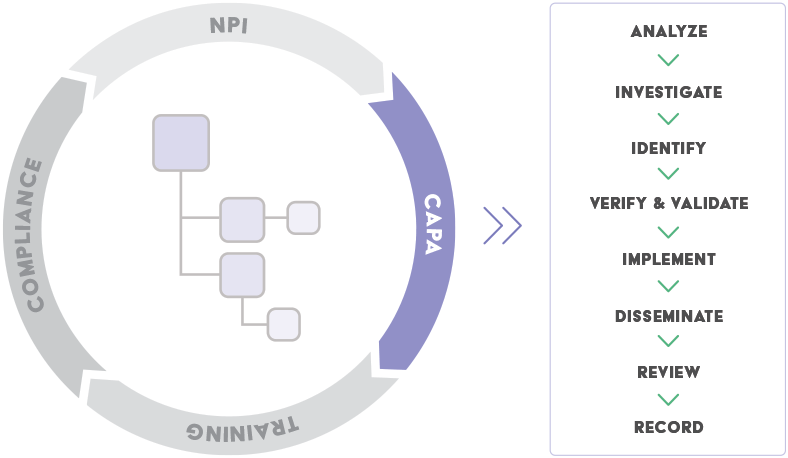
Corrective and Preventive action | CAPA management by Operon Strategist Medical Device Manufacturing Consultant - Issuu

Medical Device CAPA Process : Importance, benefits and implementation | Engineering - Johari Digital Healthcare Ltd.

Amazon.com: Principles of corrective Action and Preventive Action :CAPA: A Handbook for Quality Professionals in Medical device and Pharmaceutical Industries eBook : MUCHEMU, DAVID N.: Kindle Store




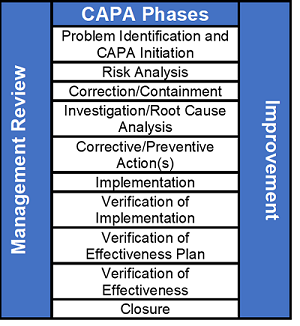
![CAPA Procedure Image[7] - Medical Device Academy Medical Device Academy CAPA Procedure Image[7] - Medical Device Academy Medical Device Academy](https://medicaldeviceacademy.com/wp-content/uploads/CAPA-Procedure-Image7.jpg)