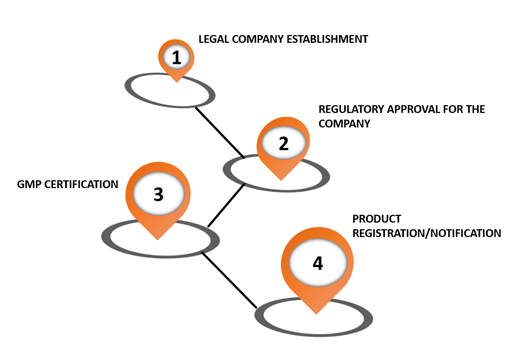
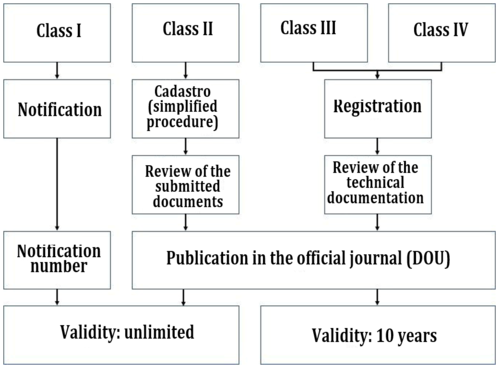
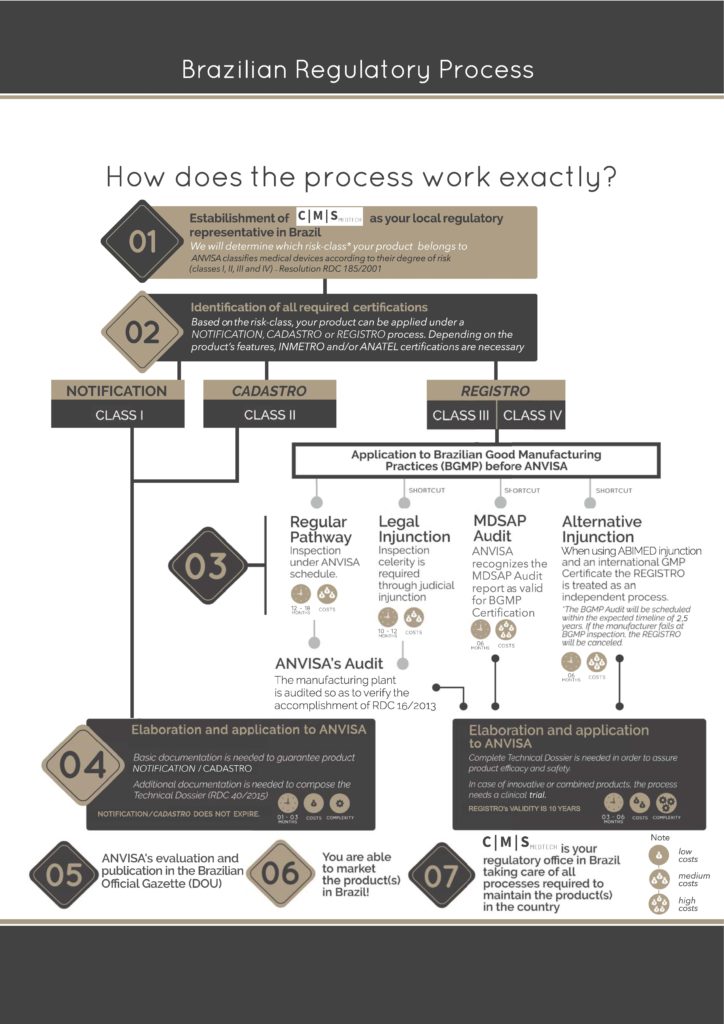
Emergo by UL on X: "Brazil's device registration process is complex, but our ANVISA chart shows the steps at a glance. Download it here: https://t.co/zIem4ne6M9 https://t.co/gUEs7gq3pg" / X

Case Study 28 : Registration of Class II Medical Device in Australia, Brazil, China, India, Pakistan and Russia