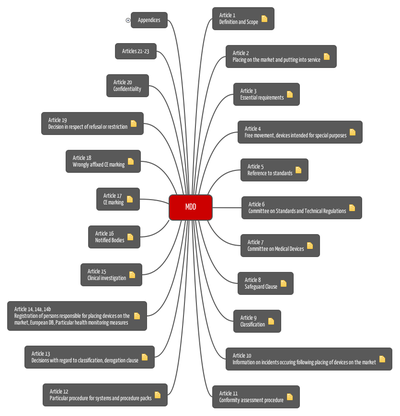
Obtaining an EC certificate confirming compliance with Directive 93/42/EEC for Medical Devices. - Telemedical Innovations

Obtaining an EC certificate confirming compliance with Directive 93/42/EEC for Medical Devices. - Telemedical Innovations

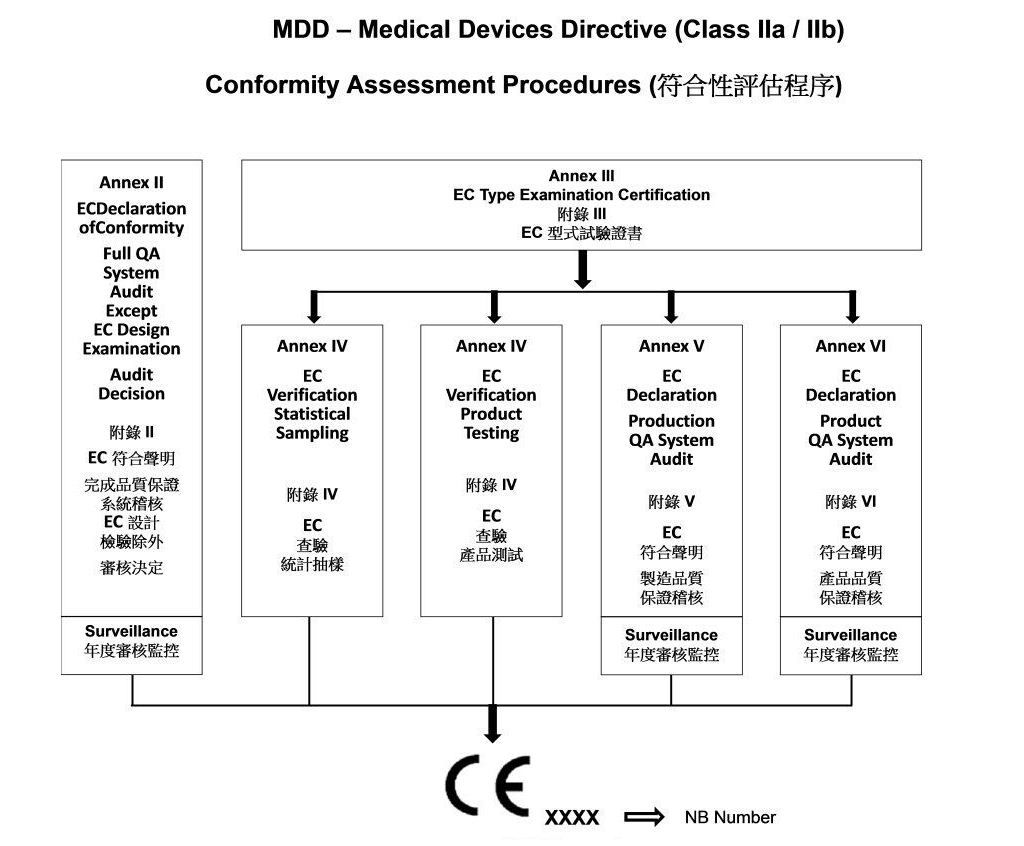
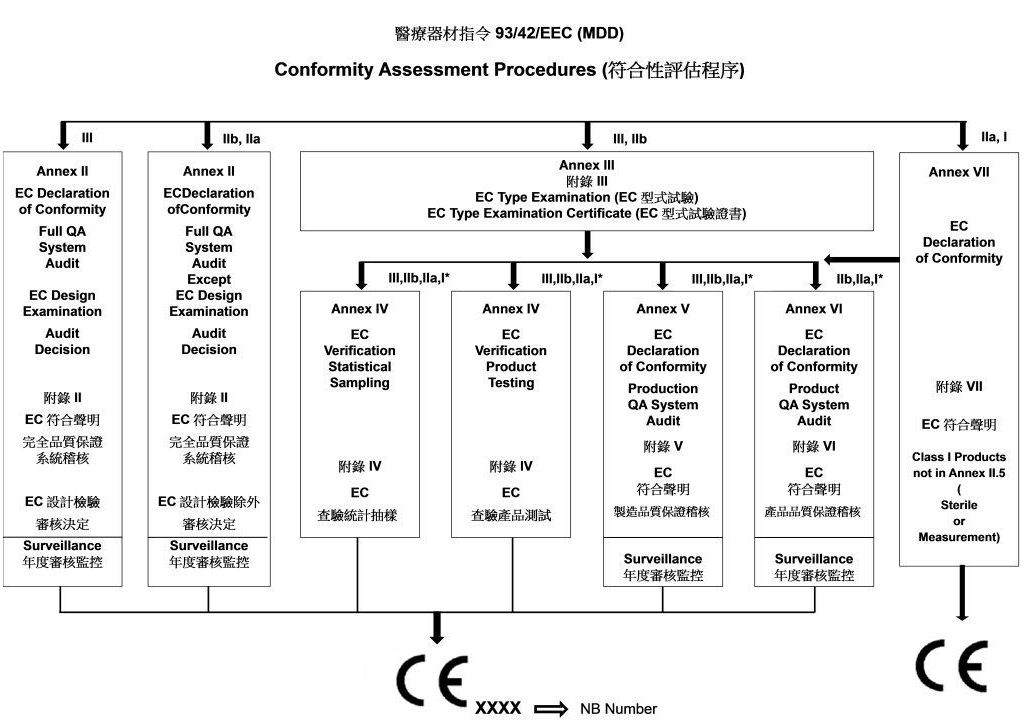
EC Certificate - Full Quality Assurance System: Directive 93/42/EEC On Medical Devices, Annex II Excluding Section 4 | PDF | Medical Device | Business

SITEC - 93/42/EEC – MEDICAL DEVICES DIRECTIVE, CE MARKING FOR EUROPE SITEC Private Limited is Certified by SGS for Directive 93/42/EEC for Class IIB and Class III Medical devices. The Certificate represents

SITEC - 93/42/EEC – MEDICAL DEVICES DIRECTIVE, CE MARKING FOR EUROPE SITEC is Certified by SGS for Directive 93/42/EEC for Class IIa Medical devices. The Certificate represents our Compliance with European Standards